Publications
Tip
Be Counted!
If you don’t see on the list below a paper you’ve written, or know of, that uses ScanImage®, please contact us!
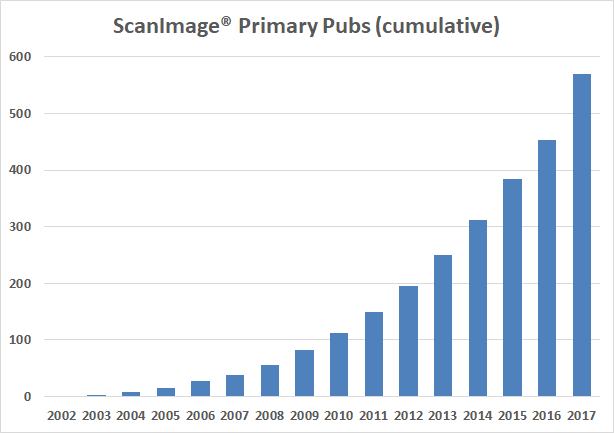
Current total research publications: 870
See Google Scholar Link for Pologruto et. al. citation_for_view
A List of some of the publications referencing use of ScanImage. For a complete list go here.
[1]M. C. Fischer, J. W. Wilson, F. E. Robles, and W. S. Warren, “Invited Review Article: Pump-probe microscopy.,” Rev Sci Instrum, vol. 87, no. 3, p. 031101, Mar. 2016.
[2]T. Lalanne, J. Oyrer, A. Mancino, E. Gregor, A. Chung, L. Huynh, S. Burwell, J. Maheux, M. Farrant, and P. J. Sjostrom, “Synapse-specific expression of calcium-permeable AMPA receptors in neocortical layer 5.,” J Physiol, vol. 594, no. 4, pp. 837–861, Feb. 2016.
[3]J. C. Tuthill and R. I. Wilson, “Supplemental Information,” Feb. 2016.
[4]W. Yang, J.-E. K. Miller, L. Carrillo-Reid, E. Pnevmatikakis, L. Paninski, R. Yuste, and D. S. Peterka, “Simultaneous Multi-plane Imaging of Neural Circuits.,” Neuron, vol. 89, no. 2, pp. 269–284, Jan. 2016.
[5]B.-J. Zandt, J. H. Liu, M. L. Veruki, and E. Hartveit, “AII amacrine cells: quantitative reconstruction and morphometric analysis of electrophysiologically identified cells in live rat retinal slices imaged with multi-photon excitation microscopy,” Brain Structure and Function, pp. 1–32, 2016.
[6]J. W. Wilson, W. S. Warren, and M. C. Fischer, “Real-time digital signal processing in multiphoton and time-resolved microscopy,” in SPIE BiOS, 2016, p. 97030O–97030O.
[7]D. Vallentin, G. Kosche, D. Lipkind, and M. A. Long, “Inhibition protects acquired song segments during vocal learning in zebra finches,” Science, vol. 351, no. 6270, pp. 267–271, 2016.
[8]P. Rupprecht, A. Prendergast, C. Wyart, and R. W. Friedrich, “Remote z-scanning with a macroscopic voice coil motor for fast 3D multiphoton laser scanning microscopy,” Biomedical Optics Express, vol. 7, no. 5, pp. 1656–1671, 2016.
[9]J. M. Rosa, S. Ruehle, H. Ding, and L. Lagnado, “Crossover Inhibition Generates Sustained Visual Responses in the Inner Retina,” Neuron, 2016.
[10]V. Phoumthipphavong, F. Barthas, S. Hassett, and A. C. Kwan, “Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal cortex,” eneuro, vol. 3, no. 2, p. ENEURO–0133, 2016.
[11]H. Y. Park and M. Song, “Visualizing mRNA Dynamics in Live Neurons and Brain Tissues,” Post-Transcriptional Gene Regulation, pp. 325–334, 2016.
[12]R. Mongeon, V. Venkatachalam, and G. Yellen, “Cytosolic NADH-NAD+ redox visualized in brain slices by two-photon fluorescence lifetime biosensor imaging,” Antioxidants & redox signaling, 2016.
[13]P. Mächler, M. T. Wyss, M. Elsayed, J. Stobart, R. Gutierrez, A. von Faber-Castell, V. Kaelin, M. Zuend, A. San Martín, I. Romero-Gómez, and others, “In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons,” Cell metabolism, vol. 23, no. 1, pp. 94–102, 2016.
[14]N. Körber and V. Stein, “In vivo imaging demonstrates dendritic spine stabilization by SynCAM 1,” Scientific Reports, vol. 6, p. 24241, 2016.
[15]L. A. Ibrahim, L. Mesik, X. Ji, Q. Fang, H. Li, Y. Li, B. Zingg, L. I. Zhang, and H. W. Tao, “Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition,” Neuron, vol. 89, no. 5, pp. 1031–1045, 2016.
[16]E. Ganmor, M. Krumin, L. F. Rossi, M. Carandini, and E. P. Simoncelli, “Direct Estimation of Firing Rates from Calcium Imaging Data,” arXiv preprint arXiv:1601.00364, 2016.
[17]G. Ferrati, F. J. Martini, and M. Maravall, “Presynaptic Adenosine Receptor-Mediated Regulation of Diverse Thalamocortical Short-Term Plasticity in the Mouse Whisker Pathway.,” Front Neural Circuits, vol. 10, p. 9, 2016.
[18]M. N. Economo, N. G. Clack, L. D. Lavis, C. R. Gerfen, K. Svoboda, E. W. Myers, and J. Chandrashekar, “A platform for brain-wide imaging and reconstruction of individual neurons.,” Elife, vol. 5, 2016.
[19]J. Cox, L. Pinto, and Y. Dan, “Calcium imaging of sleep-wake related neuronal activity in the dorsal pons.,” Nat Commun, vol. 7, p. 10763, 2016.
[20]O. Barnstedt, D. Owald, J. Felsenberg, R. Brain, J.-P. Moszynski, C. B. Talbot, P. N. Perrat, and S. Waddell, “Memory-Relevant Mushroom Body Output Synapses Are Cholinergic,” Neuron, vol. 89, no. 6, pp. 1237–1247, 2016.
[21]A. S. Bar-Noam, N. Farah, and S. Shoham, “Correction-free remotely scanned two-photon in vivo mouse retinal imaging,” Light: Science & Applications, vol. 5, no. 1, p. e16007, 2016.
[22]F. Baeza-Lehnert, S. Lengacher, B. L. Schneider, P. Aebischer, P. J. Magistretti, L. F. Barros, and B. Weber, “In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons,” Cell Metabolism, vol. 23, pp. 1–9, 2016.
[23]G. Thériault, Y. De Koninck, and N. McCarthy, Method and system for obtaining an extended-depth-of-field volumetric image using laser scanning imaging. Google Patents, 2015.
[24]S. Jayabal, L. Ljungberg, T. Erwes, A. Cormier, S. Quilez, S. El Jaouhari, and A. J. Watt, “Rapid Onset of Motor Deficits in a Mouse Model of Spinocerebellar Ataxia Type 6 Precedes Late Cerebellar Degeneration.,” eNeuro, vol. 2, no. 6, Dec. 2015.
[25]D. Sinefeld, H. P. Paudel, D. G. Ouzounov, T. G. Bifano, and C. Xu, “Adaptive optics in multiphoton microscopy: comparison of two, three and four photon fluorescence.,” Opt Express, vol. 23, no. 24, pp. 31472–31483, Nov. 2015.
[26]K. Dore, J. Aow, and R. Malinow, “Agonist binding to the NMDA receptor drives movement of its cytoplasmic domain without ion flow.,” Proc Natl Acad Sci U S A, vol. 112, no. 47, pp. 14705–14710, Nov. 2015.
[27]S. R. Cooper, M. R. Emond, P. Q. Duy, B. G. Liebau, M. A. Wolman, and J. D. Jontes, “Protocadherins control the modular assembly of neuronal columns in the zebrafish optic tectum.,” J Cell Biol, vol. 211, no. 4, pp. 807–814, Nov. 2015.
[28]J. M. Mayrhofer, F. Haiss, D. Haenni, S. Weber, M. Zuend, M. J. P. Barrett, K. D. Ferrari, P. Maechler, A. S. Saab, J. L. Stobart, M. T. Wyss, H. Johannssen, H. Osswald, L. M. Palmer, V. Revol, C.-D. Schuh, C. Urban, A. Hall, M. E. Larkum, E. Rutz-Innerhofer, H. U. Zeilhofer, U. Ziegler, and B. Weber, “Design and performance of an ultra-flexible two-photon microscope for in vivo research.,” Biomed Opt Express, vol. 6, no. 11, pp. 4228–4237, Nov. 2015.
[29]W. T. Birdsong, S. Arttamangkul, J. R. Bunzow, and J. T. Williams, “Agonist Binding and Desensitization of the mu-Opioid Receptor Is Modulated by Phosphorylation of the C-Terminal Tail Domain.,” Mol Pharmacol, vol. 88, no. 4, pp. 816–824, Oct. 2015.
[30]I. S. Stein, J. A. Gray, and K. Zito, “Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage.,” J Neurosci, vol. 35, no. 35, pp. 12303–12308, Sep. 2015.
[31]H. Makino and T. Komiyama, “Learning enhances the relative impact of top-down processing in the visual cortex.,” Nat Neurosci, vol. 18, no. 8, pp. 1116–1122, Aug. 2015.
[32]S. J. H. Park, B. G. Borghuis, P. Rahmani, Q. Zeng, I.-J. Kim, and J. B. Demb, “Function and Circuitry of VIP+ Interneurons in the Mouse Retina.,” J Neurosci, vol. 35, no. 30, pp. 10685–10700, Jul. 2015.
[33]J. H. Siegle, G. J. Hale, J. P. Newman, and J. Voigts, “Neural ensemble communities: open-source approaches to hardware for large-scale electrophysiology.,” Curr Opin Neurobiol, vol. 32, pp. 53–59, Jun. 2015.
[34]N. Chen, H. Sugihara, and M. Sur, “An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity.,” Nat Neurosci, vol. 18, no. 6, pp. 892–902, Jun. 2015.
[35]M. F. Davis, D. X. Figueroa Velez, R. P. Guevarra, M. C. Yang, M. Habeeb, M. C. Carathedathu, and S. P. Gandhi, “Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision.,” Neuron, vol. 86, no. 4, pp. 1055–1066, May 2015.
[36]J. D. Seelig and V. Jayaraman, “Neural dynamics for landmark orientation and angular path integration.,” Nature, vol. 521, no. 7551, pp. 186–191, May 2015.
[37]A. Miquelajauregui, S. Kribakaran, R. Mostany, A. Badaloni, G. G. Consalez, and C. Portera-Cailliau, “Layer 4 pyramidal neurons exhibit robust dendritic spine plasticity in vivo after input deprivation.,” J Neurosci, vol. 35, no. 18, pp. 7287–7294, May 2015.
[38]R. Srinivasan, B. S. Huang, S. Venugopal, A. D. Johnston, H. Chai, H. Zeng, P. Golshani, and B. S. Khakh, Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo., vol. 18. United States, 2015.
[39]D. Owald, J. Felsenberg, C. B. Talbot, G. Das, E. Perisse, W. Huetteroth, and S. Waddell, “Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila.,” Neuron, vol. 86, no. 2, pp. 417–427, Apr. 2015.
[40]K. B. Clancy, P. Schnepel, A. T. Rao, and D. E. Feldman, “Structure of a single whisker representation in layer 2 of mouse somatosensory cortex.,” J Neurosci, vol. 35, no. 9, pp. 3946–3958, Mar. 2015.
[41]S. C. Weber and C. P. Brangwynne, “Inverse size scaling of the nucleolus by a concentration-dependent phase transition.,” Curr Biol, vol. 25, no. 5, pp. 641–646, Mar. 2015.
[42]Y. Zhang, R. H. Cudmore, D.-T. Lin, D. J. Linden, and R. L. Huganir, “Visualization of NMDA receptor-dependent AMPA receptor synaptic plasticity in vivo.,” Nat Neurosci, vol. 18, no. 3, pp. 402–407, Mar. 2015.
[43]G. Wang, D. R. Wyskiel, W. Yang, Y. Wang, L. C. Milbern, T. Lalanne, X. Jiang, Y. Shen, Q.-Q. Sun, and J. J. Zhu, “An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits.,” Nat Protoc, vol. 10, no. 3, pp. 397–412, Mar. 2015.
[44]W. C. Oh, L. K. Parajuli, and K. Zito, “Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons.,” Cell Rep, vol. 10, no. 2, pp. 162–169, Jan. 2015.
[45]M. E. J. Sheffield and D. A. Dombeck, “Calcium transient prevalence across the dendritic arbour predicts place field properties.,” Nature, vol. 517, no. 7533, pp. 200–204, Jan. 2015.
[46]S. Arttamangkul, W. Birdsong, and J. T. Williams, “Does PKC activation increase the homologous desensitization of mu opioid receptors?,” Br J Pharmacol, vol. 172, no. 2, pp. 583–592, Jan. 2015.
[47]J. Winnubst, J. E. Cheyne, D. Niculescu, and C. Lohmann, “Spontaneous Activity Drives Local Synaptic Plasticity In Vivo,” Neuron, vol. 87, no. 2, pp. 399–410, 2015.
[48]C. D. Wilms and M. Hausser, “Reading out a spatiotemporal population code by imaging neighbouring parallel fibre axons in vivo.,” Nat Commun, vol. 6, p. 6464, 2015.
[49]W. Wang, Z. Wu, and H. Zeng, “Image distortion and its correction in linear galvanometric mirrors–based laser-scanning microscopy,” Journal of biomedical optics, vol. 20, no. 5, pp. 056001–056001, 2015.
[50]S. Wang, Z.-Y. Tsun, R. L. Wolfson, K. Shen, G. A. Wyant, M. E. Plovanich, E. D. Yuan, T. D. Jones, L. Chantranupong, W. Comb, and others, “Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1,” Science, vol. 347, no. 6218, pp. 188–194, 2015.
[51]S. Tsutsumi, M. Yamazaki, T. Miyazaki, M. Watanabe, K. Sakimura, M. Kano, and K. Kitamura, “Structure–Function Relationships between Aldolase C/Zebrin II Expression and Complex Spike Synchrony in the Cerebellum,” The Journal of Neuroscience, vol. 35, no. 2, pp. 843–852, 2015.
[52]R. Srinivasan, B. S. Huang, S. Venugopal, A. D. Johnston, H. Chai, H. Zeng, P. Golshani, and B. S. Khakh, “Ca2+ signaling in astrocytes from Ip3r2-/-mice in brain slices and during startle responses in vivo,” Nature neuroscience, vol. 18, no. 5, pp. 708–717, 2015.
[53]T. Sotelo-Hitschfeld, M. I. Niemeyer, P. Mächler, I. Ruminot, R. Lerchundi, M. T. Wyss, J. Stobart, I. Fernández-Moncada, R. Valdebenito, P. Garrido-Gerter, and others, “Channel-mediated lactate release by k+-stimulated astrocytes,” The Journal of Neuroscience, vol. 35, no. 10, pp. 4168–4178, 2015.
[54]D. Sinefeld, H. P. Paudel, D. G. Ouzounov, T. G. Bifano, and C. Xu, “Adaptive Optics in Three-Photon Fluorescence Microscopy,” in CLEO: Science and Innovations, 2015, p. STu2K–8.
[55]A. C. E. Shibata, H. K. Maebashi, Y. Nakahata, J. Nabekura, and H. Murakoshi, “Development of a molecularly evolved, highly sensitive CaMKII FRET sensor with improved expression pattern.,” PLoS One, vol. 10, no. 3, p. e0121109, 2015.
[56]C. D. Schuh, D. Haenni, E. Craigie, U. Ziegler, B. Weber, O. Devuyst, and A. M. Hall, “Long wavelength multiphoton excitation is advantageous for intravital kidney imaging,” Kidney international, 2015.
[57]M. M. Roth, J. C. Dahmen, D. R. Muir, F. Imhof, F. J. Martini, and S. B. Hofer, “Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex,” Nature neuroscience, 2015.
[58]S. A. Romano, T. Pietri, V. Pérez-Schuster, A. Jouary, M. Haudrechy, and G. Sumbre, “Spontaneous neuronal network dynamics reveal circuit’s functional adaptations for behavior,” Neuron, vol. 85, no. 5, pp. 1070–1085, 2015.
[59]B. C. Reiner and A. Dunaevsky, “Deficit in motor training-induced clustering, but not stabilization, of new dendritic spines in FMR1 knock-out mice.,” PLoS One, vol. 10, no. 5, p. e0126572, 2015.
[60]C. Pilger, H. Hachmeister, M. Müller, G. Wiebusch, and T. Huser, “SI-CARS: CARS Microscopy beyond the Diffraction Limit by Structured Illumination,” in Asia Communications and Photonics Conference, 2015, p. ASu2A–160.
[61]J. Picao-Osorio, J. Johnston, M. Landgraf, J. Berni, and C. R. Alonso, “MicroRNA-encoded behavior in Drosophila,” Science, vol. 350, no. 6262, pp. 815–820, 2015.
[62]S. P. Peron, J. Freeman, V. Iyer, C. Guo, and K. Svoboda, “A cellular resolution map of barrel cortex activity during tactile behavior,” Neuron, vol. 86, no. 3, pp. 783–799, 2015.
[63]R. Padmashri, A. Suresh, M. D. Boska, and A. Dunaevsky, “Motor-Skill Learning Is Dependent on Astrocytic Activity.,” Neural Plast, vol. 2015, p. 938023, 2015.
[64]H. Murakoshi and A. C. Shibata, “Optogenetic Imaging of Protein Activity in the Synapse Using 2-Photon Fluorescence Lifetime Imaging Microscopy,” in Optogenetics, Springer, 2015, pp. 185–197.
[65]H. Murakoshi, A. C. E. Shibata, Y. Nakahata, and J. Nabekura, “A dark green fluorescent protein as an acceptor for measurement of Forster resonance energy transfer.,” Sci Rep, vol. 5, p. 15334, 2015.
[66]R. Mostany, A. Miquelajauregui, M. Shtrahman, and C. Portera-Cailliau, “Two-photon excitation microscopy and its applications in neuroscience,” Advanced Fluorescence Microscopy: Methods and Protocols, pp. 25–42, 2015.
[67]L. Mesik, W. Ma, L. Li, L. A. Ibrahim, Z. J. Huang, L. I. Zhang, and H. W. Tao, “Functional response properties of VIP-expressing inhibitory neurons in mouse visual and auditory cortex.,” Front Neural Circuits, vol. 9, p. 22, 2015.
[68]J. M. Mayrhofer, F. Haiss, F. Helmchen, and B. Weber, “Sparse, reliable, and long-term stable representation of periodic whisker deflections in the mouse barrel cortex,” Neuroimage, vol. 115, pp. 52–63, 2015.
[69]G. Lur and M. J. Higley, “Glutamate receptor modulation is restricted to synaptic microdomains,” Cell reports, vol. 12, no. 2, pp. 326–334, 2015.
[70]S. E. Lindsey, P. G. Menon, W. J. Kowalski, A. Shekhar, H. C. Yalcin, N. Nishimura, C. B. Schaffer, J. T. Butcher, and K. Pekkan, “Growth and hemodynamics after early embryonic aortic arch occlusion,” Biomechanics and modeling in mechanobiology, vol. 14, no. 4, pp. 735–751, 2015.
[71]X. Li and Q. Wang, “3D Imaging System of Two-Photon Excitation Laser Scanning Microscopy,” in 2015 Fifth International Conference on Instrumentation and Measurement, Computer, Communication and Control (IMCCC), 2015, pp. 443–448.
[72]Y. Kozorovitskiy, R. Peixoto, W. Wang, A. Saunders, and B. L. Sabatini, “Neuromodulation of excitatory synaptogenesis in striatal development.,” Elife, vol. 4, 2015.
[73]T. Huser, H. Hachmeister, C. Pilger, V. Mönkemöller, W. Hübner, S. Hennig, M. Müller, and G. Wiebusch, “Label-free Super-resolution Optical Microscopy of Cellular Dynamics,” in Asia Communications and Photonics Conference, 2015, p. AS3I–1.
[74]M. Hashizume, T. Miyazaki, K. Sakimura, M. Watanabe, K. Kitamura, and M. Kano, “Disruption of cerebellar microzonal organization in GluD2 (GluR δ 2) knockout mouse,” Neural Circuits: Japan, 2015.
[75]M. I. Hamad, M. Krause, and P. Wahle, “Improving AM ester calcium dye loading efficiency,” Journal of neuroscience methods, vol. 240, pp. 48–60, 2015.
[76]H. Hachmeister, C. Pilger, G. Wiebusch, and T. Huser, “Enhancing the Molecular Sensitivity of Coherent Raman Scattering by Doubly-Resonant CARS (DR-CARS),” in Asia Communications and Photonics Conference, 2015, p. AM2B–3.
[77]S. C. Gantz, “Leveraging the occurrence of spontaneous D2 receptor-mediated IPSCs to understand the dopamine synapse,” 2015.
[78]S. C. Gantz, B. G. Robinson, D. C. Buck, J. R. Bunzow, R. L. Neve, J. T. Williams, and K. A. Neve, “Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium.,” Elife, vol. 4, 2015.
[79]M. G. Frantz, R. J. Kast, H. M. Dorton, K. S. Chapman, and A. W. McGee, “Nogo receptor 1 limits ocular dominance plasticity but not turnover of axonal boutons in a model of amblyopia,” Cerebral Cortex, p. bhv014, 2015.
[80]B. F. Fosque, Y. Sun, H. Dana, C.-T. Yang, T. Ohyama, M. R. Tadross, R. Patel, M. Zlatic, D. S. Kim, M. B. Ahrens, and others, “Labeling of active neural circuits in vivo with designed calcium integrators,” Science, vol. 347, no. 6223, pp. 755–760, 2015.
[81]P. Ferrand, “GPScan. VI: A general-purpose LabVIEW program for scanning imaging or any application requiring synchronous analog voltage generation and data acquisition,” Computer Physics Communications, vol. 192, pp. 342–347, 2015.
[82]O. H. Do, J. T. Low, and P. Thorn, “Leprdb Mouse Model of Type 2 Diabetes: Pancreatic Islet Isolation and Live-cell 2-Photon Imaging Of Intact Islets,” JoVE (Journal of Visualized Experiments), no. 99, pp. e52632–e52632, 2015.
[83]M. Collot, C. D. Wilms, A. Bentkhayet, P. Marcaggi, K. Couchman, S. Charpak, S. Dieudonne, M. Hausser, A. Feltz, and J.-M. Mallet, “CaRuby-Nano: a novel high affinity calcium probe for dual color imaging.,” Elife, vol. 4, 2015.
[84]P. Coiro, R. Padmashri, A. Suresh, E. Spartz, G. Pendyala, S. Chou, Y. Jung, B. Meays, S. Roy, N. Gautam, and others, “Impaired synaptic development in a maternal immune activation mouse model of neurodevelopmental disorders,” Brain, behavior, and immunity, vol. 50, pp. 249–258, 2015.
[85]Á. Castilho, A. F. Ambrósio, E. Hartveit, and M. L. Veruki, “Disruption of a neural microcircuit in the rod pathway of the mammalian retina by diabetes mellitus,” The Journal of Neuroscience, vol. 35, no. 13, pp. 5422–5433, 2015.
[86]C. Blumer, C. Vivien, C. Genoud, A. Perez-Alvarez, J. S. Wiegert, T. Vetter, and T. G. Oertner, “Automated analysis of spine dynamics on live CA1 pyramidal cells,” Medical image analysis, vol. 19, no. 1, pp. 87–97, 2015.
[87]W. T. Birdsong, S. Arttamangkul, J. R. Bunzow, and J. T. Williams, “Agonist binding and desensitization of the μ-opioid receptor is modulated by phosphorylation of the C-terminal tail domain,” Molecular pharmacology, vol. 88, no. 4, pp. 816–824, 2015.
[88]A. J. Barker and H. Baier, “Sensorimotor Decision Making in the Zebrafish Tectum,” Current Biology, vol. 25, no. 21, pp. 2804–2814, 2015.
[89]S. Arttamangkul, W. Birdsong, and J. T. Williams, “Does PKC activation increase the homologous desensitization of μ opioid receptors?,” British journal of pharmacology, vol. 172, no. 2, pp. 583–592, 2015.
[90]D. A. Fortin, S. E. Tillo, G. Yang, J.-C. Rah, J. B. Melander, S. Bai, O. Soler-Cedeno, M. Qin, B. V. Zemelman, C. Guo, T. Mao, and H. Zhong, “Live imaging of endogenous PSD-95 using ENABLED: a conditional strategy to fluorescently label endogenous proteins.,” J Neurosci, vol. 34, no. 50, pp. 16698–16712, Dec. 2014.
[91]J. G. Heys, K. V. Rangarajan, and D. A. Dombeck, “The functional micro-organization of grid cells revealed by cellular-resolution imaging.,” Neuron, vol. 84, no. 5, pp. 1079–1090, Dec. 2014.
[92]Y. T. Tang, J. M. Mendez, J. J. Theriot, P. M. Sawant, H. E. Lopez-Valdes, Y. S. Ju, and K. C. Brennan, “Minimum conditions for the induction of cortical spreading depression in brain slices.,” J Neurophysiol, vol. 112, no. 10, pp. 2572–2579, Nov. 2014.
[93]J. Johnston, H. Ding, S. H. Seibel, F. Esposti, and L. Lagnado, “Rapid mapping of visual receptive fields by filtered back projection: application to multi-neuronal electrophysiology and imaging.,” J Physiol, vol. 592, no. 22, pp. 4839–4854, Nov. 2014.
[94]S. Lin, D. Owald, V. Chandra, C. Talbot, W. Huetteroth, and S. Waddell, “Neural correlates of water reward in thirsty Drosophila.,” Nat Neurosci, vol. 17, no. 11, pp. 1536–1542, Nov. 2014.
[95]T. Baden, A. Nikolaev, F. Esposti, E. Dreosti, B. Odermatt, and L. Lagnado, “A synaptic mechanism for temporal filtering of visual signals.,” PLoS Biol, vol. 12, no. 10, p. e1001972, Oct. 2014.
[96]K. M. Werner, L. J. Perez, R. Ghosh, M. F. Semmelhack, and B. L. Bassler, “Caenorhabditis elegans recognizes a bacterial quorum-sensing signal molecule through the AWCON neuron.,” J Biol Chem, vol. 289, no. 38, pp. 26566–26573, Sep. 2014.
[97]T. R. Thiele, J. C. Donovan, and H. Baier, “Descending control of swim posture by a midbrain nucleus in zebrafish.,” Neuron, vol. 83, no. 3, pp. 679–691, Aug. 2014.
[98]E. R. Schreiter, L. L. Looger, and B. F. Fosque, Fluorescent protein-based indicators. Google Patents, 2014.
[99]M. Xue, B. V. Atallah, and M. Scanziani, “Equalizing excitation-inhibition ratios across visual cortical neurons.,” Nature, vol. 511, no. 7511, pp. 596–600, Jul. 2014.
[100]J. Elstrott, K. B. Clancy, H. Jafri, I. Akimenko, and D. E. Feldman, “Cellular mechanisms for response heterogeneity among L2/3 pyramidal cells in whisker somatosensory cortex.,” J Neurophysiol, vol. 112, no. 2, pp. 233–248, Jul. 2014.
[101]K. M. Seemann and B. Kuhn, “Multi-photon excited luminescence of magnetic FePt core-shell nanoparticles.,” Biomed Opt Express, vol. 5, no. 7, pp. 2446–2457, Jul. 2014.
[102]E. A. Calle, S. Vesuna, S. Dimitrievska, K. Zhou, A. Huang, L. Zhao, L. E. Niklason, and M. J. Levene, “The use of optical clearing and multiphoton microscopy for investigation of three-dimensional tissue-engineered constructs.,” Tissue Eng Part C Methods, vol. 20, no. 7, pp. 570–577, Jul. 2014.
[103]M. Paukert, A. Agarwal, J. Cha, V. A. Doze, J. U. Kang, and D. E. Bergles, “Norepinephrine controls astroglial responsiveness to local circuit activity.,” Neuron, vol. 82, no. 6, pp. 1263–1270, Jun. 2014.
[104]S. E. Crowe and G. C. R. Ellis-Davies, “Longitudinal in vivo two-photon fluorescence imaging.,” J Comp Neurol, vol. 522, no. 8, pp. 1708–1727, Jun. 2014.
[105]M. Tada, A. Takeuchi, M. Hashizume, K. Kitamura, and M. Kano, “A highly sensitive fluorescent indicator dye for calcium imaging of neural activity in vitro and in vivo.,” Eur J Neurosci, vol. 39, no. 11, pp. 1720–1728, Jun. 2014.
[106]K. B. Clancy, A. C. Koralek, R. M. Costa, D. E. Feldman, and J. M. Carmena, “Volitional modulation of optically recorded calcium signals during neuroprosthetic learning.,” Nat Neurosci, vol. 17, no. 6, pp. 807–809, Jun. 2014.
[107]M. L. Viger, W. Sheng, K. Dore, A. H. Alhasan, C.-J. Carling, J. Lux, C. de Gracia Lux, M. Grossman, R. Malinow, and A. Almutairi, “Near-infrared-induced heating of confined water in polymeric particles for efficient payload release.,” ACS Nano, vol. 8, no. 5, pp. 4815–4826, May 2014.
[108]B. G. Borghuis, L. L. Looger, S. Tomita, and J. B. Demb, “Kainate receptors mediate signaling in both transient and sustained OFF bipolar cell pathways in mouse retina.,” J Neurosci, vol. 34, no. 18, pp. 6128–6139, Apr. 2014.
[109]S. J. Kuhlman, D. H. O’Connor, K. Fox, and K. Svoboda, “Structural plasticity within the barrel cortex during initial phases of whisker-dependent learning.,” J Neurosci, vol. 34, no. 17, pp. 6078–6083, Apr. 2014.
[110]B. He, K. Doubrovinski, O. Polyakov, and E. Wieschaus, “Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation.,” Nature, vol. 508, no. 7496, pp. 392–396, Apr. 2014.
[111]A. Cruz-Martin, R. N. El-Danaf, F. Osakada, B. Sriram, O. S. Dhande, P. L. Nguyen, E. M. Callaway, A. Ghosh, and A. D. Huberman, “A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex.,” Nature, vol. 507, no. 7492, pp. 358–361, Mar. 2014.
[112]S. J. H. Park, I.-J. Kim, L. L. Looger, J. B. Demb, and B. G. Borghuis, “Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning.,” J Neurosci, vol. 34, no. 11, pp. 3976–3981, Mar. 2014.
[113]T.-M. Wang, L. C. Holzhausen, and R. H. Kramer, “Imaging an optogenetic pH sensor reveals that protons mediate lateral inhibition in the retina.,” Nat Neurosci, vol. 17, no. 2, pp. 262–268, Feb. 2014.
[114]M. Fisek and R. I. Wilson, “Stereotyped connectivity and computations in higher-order olfactory neurons.,” Nat Neurosci, vol. 17, no. 2, pp. 280–288, Feb. 2014.
[115]A. F. Oliveira and R. Yasuda, “Neurofibromin is the major ras inactivator in dendritic spines.,” J Neurosci, vol. 34, no. 3, pp. 776–783, Jan. 2014.
[116]J. A. Murphy, I. S. Stein, C. G. Lau, R. T. Peixoto, T. K. Aman, N. Kaneko, K. Aromolaran, J. L. Saulnier, G. K. Popescu, B. L. Sabatini, J. W. Hell, and R. S. Zukin, “Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines.,” J Neurosci, vol. 34, no. 3, pp. 869–879, Jan. 2014.
[117]M. Zou, P. De Koninck, R. L. Neve, and R. W. Friedrich, “Fast gene transfer into the adult zebrafish brain by herpes simplex virus 1 (HSV-1) and electroporation: methods and optogenetic applications.,” Front Neural Circuits, vol. 8, p. 41, 2014.
[118]J. A. Wright, T. Richards, and S. K. Srai, “The role of iron in the skin and cutaneous wound healing,” Frontiers in pharmacology, vol. 5, 2014.
[119]N. X. Tritsch, W.-J. Oh, C. Gu, and B. L. Sabatini, “Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis.,” Elife, vol. 3, p. e01936, 2014.
[120]C. Tischer, V. Hilsenstein, K. Hanson, and R. Pepperkok, “Adaptive fluorescence microscopy by online feedback image analysis,” Methods in cell biology, vol. 123, pp. 489–503, 2014.
[121]P. Thorn, “Measurement of Dynamic F-Actin Changes During Exocytosis,” Exocytosis and Endocytosis, pp. 423–431, 2014.
[122]R. Tatti, K. Bhaukaurally, O. Gschwend, R. P. Seal, R. H. Edwards, I. Rodriguez, and A. Carleton, “A population of glomerular glutamatergic neurons controls sensory information transfer in the mouse olfactory bulb.,” Nat Commun, vol. 5, p. 3791, 2014.
[123]C. Straub, A. J. Granger, J. L. Saulnier, and B. L. Sabatini, “CRISPR/Cas9-mediated gene knock-down in post-mitotic neurons.,” PLoS One, vol. 9, no. 8, p. e105584, 2014.
[124]P. Soda, “BioImage Informatics: the challenge of knowledge extraction from biological images,” in Digital Technologies (DT), 2014 10th International Conference on, 2014, pp. 311–320.
[125]T. P. Santisakultarm, C. Q. Paduano, T. Stokol, T. L. Southard, N. Nishimura, R. C. Skoda, W. L. Olbricht, A. I. Schafer, R. T. Silver, and C. B. Schaffer, “Stalled cerebral capillary blood flow in mouse models of essential thrombocythemia and polycythemia vera revealed by in vivo two-photon imaging,” Journal of Thrombosis and Haemostasis, vol. 12, no. 12, pp. 2120–2130, 2014.
[126]J. I. Sanders and A. Kepecs, “A low-cost programmable pulse generator for physiology and behavior.,” Front Neuroeng, vol. 7, p. 43, 2014.
[127]M. Rothermel and M. Wachowiak, “Functional imaging of cortical feedback projections to the olfactory bulb.,” Front Neural Circuits, vol. 8, p. 73, 2014.
[128]J. A. Rosenthal, C.-J. Huang, A. M. Doody, T. Leung, K. Mineta, D. D. Feng, E. C. Wayne, N. Nishimura, C. Leifer, M. P. DeLisa, S. Mendez, and D. Putnam, “Mechanistic insight into the TH1-biased immune response to recombinant subunit vaccines delivered by probiotic bacteria-derived outer membrane vesicles.,” PLoS One, vol. 9, no. 11, p. e112802, 2014.
[129]D. G. Rosenegger, C. H. T. Tran, J. LeDue, N. Zhou, and G. R. Gordon, “A high performance, cost-effective, open-source microscope for scanning two-photon microscopy that is modular and readily adaptable.,” PLoS One, vol. 9, no. 10, p. e110475, 2014.
[130]E. Robles, E. Laurell, and H. Baier, “The retinal projectome reveals brain-area-specific visual representations generated by ganglion cell diversity,” Current Biology, vol. 24, no. 18, pp. 2085–2096, 2014.
[131]J. P. Rickgauer, K. Deisseroth, and D. W. Tank, “Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields,” Nature neuroscience, vol. 17, no. 12, pp. 1816–1824, 2014.
[132]H. Peng, J. Tang, H. Xiao, A. Bria, J. Zhou, V. Butler, Z. Zhou, P. T. Gonzalez-Bellido, S. W. Oh, J. Chen, A. Mitra, R. W. Tsien, H. Zeng, G. A. Ascoli, G. Iannello, M. Hawrylycz, E. Myers, and F. Long, “Virtual finger boosts three-dimensional imaging and microsurgery as well as terabyte volume image visualization and analysis.,” Nat Commun, vol. 5, p. 4342, 2014.
[133]J. I. Park, M. G. Frantz, R. J. Kast, K. S. Chapman, H. M. Dorton, C.-E. Stephany, M. T. Arnett, D. H. Herman, and A. W. McGee, “Nogo receptor 1 limits tactile task performance independent of basal anatomical plasticity.,” PLoS One, vol. 9, no. 11, p. e112678, 2014.
[134]H. Y. Park, H. Lim, Y. J. Yoon, A. Follenzi, C. Nwokafor, M. Lopez-Jones, X. Meng, and R. H. Singer, “Visualization of dynamics of single endogenous mRNA labeled in live mouse,” Science, vol. 343, no. 6169, pp. 422–424, 2014.
[135]F. Najafi, “Trial-by-trial coding of instructive signals in the cerebellum: Insights from eyeblink conditioning in mice,” 2014.
[136]F. Najafi, A. Giovannucci, S. S.-H. Wang, and J. F. Medina, “Coding of stimulus strength via analog calcium signals in Purkinje cell dendrites of awake mice.,” Elife, vol. 3, p. e03663, 2014.
[137]L. B. Mostaço-Guidolin, E. K. Kohlenberg, M. Smith, M. Hewko, A. Major, M. G. Sowa, and A. C.-T. Ko, “Quantitative nonlinear optical assessment of atherosclerosis progression in rabbits,” Analytical chemistry, vol. 86, no. 13, pp. 6346–6354, 2014.
[138]J. Lecoq, J. Savall, D. Vucinic, B. F. Grewe, H. Kim, J. Z. Li, L. J. Kitch, and M. J. Schnitzer, “Visualizing mammalian brain area interactions by dual-axis two-photon calcium imaging,” Nature neuroscience, vol. 17, no. 12, pp. 1825–1829, 2014.
[139]A. C. Koralek, “Large-Scale Neuronal Network Changes Underlying Neuroprosthetic Learning,” 2014.
[140]C. K. Kim, A. Miri, L. C. Leung, A. Berndt, P. Mourrain, D. W. Tank, and R. D. Burdine, “Prolonged, brain-wide expression of nuclear-localized GCaMP3 for functional circuit mapping.,” Front Neural Circuits, vol. 8, p. 138, 2014.
[141]D. M. Huland, D. G. Ouzounov, D. R. Rivera, C. M. Brown, and C. Xu, “Intravital Multiphoton Endoscopy,” in Advances in Intravital Microscopy, Springer, 2014, pp. 305–370.
[142]E. Hopp, A. Borst, and J. Haag, “Subcellular mapping of dendritic activity in optic flow processing neurons,” Journal of Comparative Physiology A, vol. 200, no. 5, pp. 359–370, 2014.
[143]M. I. Hamad, A. Jack, O. Klatt, M. Lorkowski, T. Strasdeit, S. Kott, C. Sager, M. Hollmann, and P. Wahle, “Type I TARPs promote dendritic growth of early postnatal neocortical pyramidal cells in organotypic cultures,” Development, vol. 141, no. 8, pp. 1737–1748, 2014.
[144]J. M. Gee, N. A. Smith, F. R. Fernandez, M. N. Economo, D. Brunert, M. Rothermel, S. C. Morris, A. Talbot, S. Palumbos, J. M. Ichida, and others, “Imaging activity in neurons and glia with a Polr2a-based and cre-dependent GCaMP5G-IRES-tdTomato reporter mouse,” Neuron, vol. 83, no. 5, pp. 1058–1072, 2014.
[145]F. Gambino, S. Pagès, V. Kehayas, D. Baptista, R. Tatti, A. Carleton, and A. Holtmaat, “Sensory-evoked LTP driven by dendritic plateau potentials in vivo,” Nature, vol. 515, no. 7525, pp. 116–119, 2014.
[146]J. Freeman, N. Vladimirov, T. Kawashima, Y. Mu, N. J. Sofroniew, D. V. Bennett, J. Rosen, C.-T. Yang, L. L. Looger, and M. B. Ahrens, “Mapping brain activity at scale with cluster computing,” Nature methods, vol. 11, no. 9, pp. 941–950, 2014.
[147]M. Fisek, “Connectivity and computations in higher-order olfactory neurons in Drosophila,” 2014.
[148]H. Dana, A. Marom, S. Paluch, R. Dvorkin, I. Brosh, and S. Shoham, “Hybrid multiphoton volumetric functional imaging of large-scale bioengineered neuronal networks.,” Nat Commun, vol. 5, p. 3997, 2014.
[149]H. Dana, T.-W. Chen, A. Hu, B. C. Shields, C. Guo, L. L. Looger, D. S. Kim, and K. Svoboda, “Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo.,” PLoS One, vol. 9, no. 9, p. e108697, 2014.
[150]A. Cupido, B. Catalin, H. Steffens, and F. Kirchhoff, “Surgical Procedures to Study Microglial Motility in the Brain and in the Spinal Cord by In Vivo Two-Photon Laser-Scanning Microscopy,” Laser scanning microscopy and quantitative image analysis of neuronal tissue, pp. 37–50, 2014.
[151]A. Cruz-Martin and C. Portera-Cailliau, “In vivo imaging of axonal and dendritic structures in neonatal mouse cortex,” Cold Spring Harbor Protocols, vol. 2014, no. 1, p. pdb–prot080150, 2014.
[152]K. B. Clancy, “Using calcium imaging to understand function and learning in layer 2/3 of cerebral cortex,” University of California, Berkeley, 2014.
[153]Y. Chen, J. L. Saulnier, G. Yellen, and B. L. Sabatini, “A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging,” 2014.
[154]M. Cane, B. Maco, G. Knott, and A. Holtmaat, “The relationship between PSD-95 clustering and spine stability in vivo,” The Journal of Neuroscience, vol. 34, no. 6, pp. 2075–2086, 2014.
[155]R. A. A. Campbell, R. W. Eifert, and G. C. Turner, “OpenStage: a low-cost motorized microscope stage with sub-micron positioning accuracy.,” PLoS One, vol. 9, no. 2, p. e88977, 2014.
[156]L. Campagnola, M. B. Kratz, and P. B. Manis, “ACQ4: an open-source software platform for data acquisition and analysis in neurophysiology research.,” Front Neuroinform, vol. 8, p. 3, 2014.
[157]A. V. Blackman, S. Grabuschnig, R. Legenstein, and P. J. Sjostrom, “A comparison of manual neuronal reconstruction from biocytin histology or,” Front Neuroanat, vol. 8, p. 65, 2014.
[158]B. Biermann, S. Sokoll, J. Klueva, M. Missler, J. S. Wiegert, J.-B. Sibarita, and M. Heine, “Imaging of molecular surface dynamics in brain slices using single-particle tracking.,” Nat Commun, vol. 5, p. 3024, 2014.
[159]S. Arunkarthick, M. M. Bijeesh, A. S. Vetcha, N. Rastogi, P. Nandakumar, and G. K. Varier, “Design and construction of a confocal laser scanning microscope for biomolecular imaging,” CURRENT SCIENCE, vol. 107, no. 12, p. 1965, 2014.
[160]K. S. Sinsimer, J. J. Lee, S. Y. Thiberge, and E. R. Gavis, “Germ plasm anchoring is a dynamic state that requires persistent trafficking.,” Cell Rep, vol. 5, no. 5, pp. 1169–1177, Dec. 2013.
[161]R. Padmashri, B. C. Reiner, A. Suresh, E. Spartz, and A. Dunaevsky, “Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome.,” J Neurosci, vol. 33, no. 50, pp. 19715–19723, Dec. 2013.
[162]E.-M. Schotz, M. Lanio, J. A. Talbot, and M. L. Manning, “Glassy dynamics in three-dimensional embryonic tissues.,” J R Soc Interface, vol. 10, no. 89, p. 20130726, Dec. 2013.
[163]R. G. Fernandez and A. T. De Computadores, “Estudios De Ingenieria en Informatica,” Dec. 2013.
[164]S. Zhai, E. D. Ark, P. Parra-Bueno, and R. Yasuda, “Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines.,” Science, vol. 342, no. 6162, pp. 1107–1111, Nov. 2013.
[165]M. L. Andermann, N. B. Gilfoy, G. J. Goldey, R. N. S. Sachdev, M. Wolfel, D. A. McCormick, R. C. Reid, and M. J. Levene, “Chronic cellular imaging of entire cortical columns in awake mice using microprisms.,” Neuron, vol. 80, no. 4, pp. 900–913, Nov. 2013.
[166]J. S. Wiegert and T. G. Oertner, “Long-term depression triggers the selective elimination of weakly integrated synapses.,” Proc Natl Acad Sci U S A, vol. 110, no. 47, pp. E4510–4519, Nov. 2013.
[167]J. D. Seelig and V. Jayaraman, “Feature detection and orientation tuning in the Drosophila central complex,” Nature, vol. 503, no. 7475, pp. 262–266, Nov. 2013.
[168]P. Garcia-Junco-Clemente, D. K. Chow, E. Tring, M. T. Lazaro, J. T. Trachtenberg, and P. Golshani, “Overexpression of calcium-activated potassium channels underlies cortical dysfunction in a model of PTEN-associated autism.,” Proc Natl Acad Sci U S A, vol. 110, no. 45, pp. 18297–18302, Nov. 2013.
[169]B. B. Scott, C. D. Brody, and D. W. Tank, “Cellular resolution functional imaging in behaving rats using voluntary head restraint,” Neuron, vol. 80, no. 2, pp. 371–384, Oct. 2013.
[170]B. Nahir and C. E. Jahr, “Activation of extrasynaptic NMDARs at individual parallel fiber-molecular layer interneuron synapses in cerebellum.,” J Neurosci, vol. 33, no. 41, pp. 16323–16333, Oct. 2013.
[171]S. J. Kuhlman, N. D. Olivas, E. Tring, T. Ikrar, X. Xu, and J. T. Trachtenberg, “A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex.,” Nature, vol. 501, no. 7468, pp. 543–546, Sep. 2013.
[172]A. E. Granstedt, J. B. Bosse, S. Y. Thiberge, and L. W. Enquist, “In vivo imaging of alphaherpesvirus infection reveals synchronized activity dependent on axonal sorting of viral proteins.,” Proc Natl Acad Sci U S A, vol. 110, no. 37, pp. E3516–3525, Sep. 2013.
[173]P.-O. Polack, J. Friedman, and P. Golshani, “Cellular mechanisms of brain state-dependent gain modulation in visual cortex.,” Nat Neurosci, vol. 16, no. 9, pp. 1331–1339, Sep. 2013.
[174]P. S. Holcomb, B. K. Hoffpauir, M. C. Hoyson, D. R. Jackson, T. J. Deerinck, G. S. Marrs, M. Dehoff, J. Wu, M. H. Ellisman, and G. A. Spirou, “Synaptic inputs compete during rapid formation of the calyx of Held: a new model system for neural development.,” J Neurosci, vol. 33, no. 32, pp. 12954–12969, Aug. 2013.
[175]J. Onativia, S. R. Schultz, and P. L. Dragotti, “A finite rate of innovation algorithm for fast and accurate spike detection from two-photon calcium imaging.,” J Neural Eng, vol. 10, no. 4, p. 046017, Aug. 2013.
[176]T.-W. Chen, T. J. Wardill, Y. Sun, S. R. Pulver, S. L. Renninger, A. Baohan, E. R. Schreiter, R. A. Kerr, M. B. Orger, V. Jayaraman, L. L. Looger, K. Svoboda, and D. S. Kim, “Ultrasensitive fluorescent proteins for imaging neuronal activity,” Nature, vol. 499, no. 7458, pp. 295–300, Jul. 2013.
[177]F. Esposti, J. Johnston, J. M. Rosa, K.-M. Leung, and L. Lagnado, “Olfactory stimulation selectively modulates the OFF pathway in the retina of zebrafish.,” Neuron, vol. 79, no. 1, pp. 97–110, Jul. 2013.
[178]J. T. Goncalves, J. E. Anstey, P. Golshani, and C. Portera-Cailliau, “Circuit level defects in the developing neocortex of Fragile X mice.,” Nat Neurosci, vol. 16, no. 7, pp. 903–909, Jul. 2013.
[179]B. Catalin, S. Mitran, C. Albu, and M. Iancau, “Comparative aspects of microglia reaction in white and gray matter.,” Curr Health Sci J, vol. 39, no. 3, pp. 151–154, Jul. 2013.
[180]N. R. Wilson, J. Schummers, C. A. Runyan, S. X. Yan, R. E. Chen, Y. Deng, and M. Sur, “Two-way communication with neural networks in vivo using focused light.,” Nat Protoc, vol. 8, no. 6, pp. 1184–1203, Jun. 2013.
[181]E. R. Schneider, E. F. Civillico, and S. S.-H. Wang, “Calcium-based dendritic excitability and its regulation in the deep cerebellar nuclei.,” J Neurophysiol, vol. 109, no. 9, pp. 2282–2292, May 2013.
[182]R. Bock, J. H. Shin, A. R. Kaplan, A. Dobi, E. Markey, P. F. Kramer, C. M. Gremel, C. H. Christensen, M. F. Adrover, and V. A. Alvarez, “Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use.,” Nat Neurosci, vol. 16, no. 5, pp. 632–638, May 2013.
[183]D. G. Johnston, M. Denizet, R. Mostany, and C. Portera-Cailliau, “Chronic in vivo imaging shows no evidence of dendritic plasticity or functional remapping in the contralesional cortex after stroke.,” Cereb Cortex, vol. 23, no. 4, pp. 751–762, Apr. 2013.
[184]M. Wachowiak, M. N. Economo, M. Diaz-Quesada, D. Brunert, D. W. Wesson, J. A. White, and M. Rothermel, “Optical dissection of odor information processing in vivo using GCaMPs expressed in specified cell types of the olfactory bulb.,” J Neurosci, vol. 33, no. 12, pp. 5285–5300, Mar. 2013.
[185]E. M. Szatmari, A. F. Oliveira, E. J. Sumner, and R. Yasuda, “Centaurin-alpha1-Ras-Elk-1 signaling at mitochondria mediates beta-amyloid-induced synaptic dysfunction.,” J Neurosci, vol. 33, no. 12, pp. 5367–5374, Mar. 2013.
[186]E. Robles, A. Filosa, and H. Baier, “Precise lamination of retinal axons generates multiple parallel input pathways in the tectum.,” J Neurosci, vol. 33, no. 11, pp. 5027–5039, Mar. 2013.
[187]N. G. Horton, K. Wang, D. Kobat, C. G. Clark, F. W. Wise, C. B. Schaffer, and C. Xu, “three-photon microscopy of subcortical structures within an intact mouse brain.,” Nat Photonics, vol. 7, no. 3, Mar. 2013.
[188]K. T. Takasaki, J. B. Ding, and B. L. Sabatini, “Live-cell superresolution imaging by pulsed STED two-photon excitation microscopy.,” Biophys J, vol. 104, no. 4, pp. 770–777, Feb. 2013.
[189]W. C. Oh, T. C. Hill, and K. Zito, “Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening.,” Proc Natl Acad Sci U S A, vol. 110, no. 4, pp. E305–312, Jan. 2013.
[190]P. Zhu, T. Frank, and R. W. Friedrich, “Equalization of odor representations by a network of electrically coupled inhibitory interneurons,” Nature neuroscience, vol. 16, no. 11, pp. 1678–1686, 2013.
[191]R. Weber, “Nonlinear microscopy for material characterization,” 2013.
[192]F. Wang, A. Saiz-Lopez, A. S. Mahajan, J. C. G. Martín, D. Armstrong, M. Lemes, T. Hay, and C. Prados-Roman, “Supplementary Material for,” 2013.
[193]G. Thériault, Y. De Koninck, and N. McCarthy, “Extended depth of field microscopy for rapid volumetric two-photon imaging,” Optics express, vol. 21, no. 8, pp. 10095–10104, 2013.
[194]J. C. Tang, T. Szikra, Y. Kozorovitskiy, M. Teixiera, B. L. Sabatini, B. Roska, and C. L. Cepko, “A nanobody-based system using fluorescent proteins as scaffolds for cell-specific gene manipulation,” Cell, vol. 154, no. 4, pp. 928–939, 2013.
[195]E. M. Szatmari, A. F. Oliveira, E. J. Sumner, and R. Yasuda, “Centaurin-α1-Ras-Elk-1 signaling at mitochondria mediates β-amyloid-induced synaptic dysfunction,” The Journal of Neuroscience, vol. 33, no. 12, pp. 5367–5374, 2013.
[196]X. R. Sun, A. Badura, D. A. Pacheco, L. A. Lynch, E. R. Schneider, M. P. Taylor, I. B. Hogue, L. W. Enquist, M. Murthy, and S. S.-H. Wang, “Fast GCaMPs for improved tracking of neuronal activity.,” Nat Commun, vol. 4, p. 2170, 2013.
[197]S. L. Smith, I. T. Smith, T. Branco, and M. Häusser, “Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo,” Nature, vol. 503, no. 7474, pp. 115–120, 2013.
[198]V. Schubert, D. Lebrecht, and A. Holtmaat, “Peripheral deafferentation-driven functional somatosensory map shifts are associated with local, not large-scale dendritic structural plasticity,” The Journal of Neuroscience, vol. 33, no. 22, pp. 9474–9487, 2013.
[199]I. W. Schie, L. Nolte, T. L. Pedersen, Z. Smith, J. Wu, I. Yahiatene, J. W. Newman, and T. Huser, “Direct comparison of fatty acid ratios in single cellular lipid droplets as determined by comparative Raman spectroscopy and gas chromatography,” Analyst, vol. 138, no. 21, pp. 6662–6670, 2013.
[200]T. Rose, P. Schoenenberger, K. Jezek, and T. G. Oertner, “Developmental refinement of vesicle cycling at Schaffer collateral synapses,” Neuron, vol. 77, no. 6, pp. 1109–1121, 2013.
[201]S. L. Renninger and M. B. Orger, “Two-photon imaging of neural population activity in zebrafish,” Methods, vol. 62, no. 3, pp. 255–267, 2013.
[202]F. J. Probst, R. R. Corrigan, D. Del Gaudio, A. P. Salinger, I. Lorenzo, S. S. Gao, I. Chiu, A. Xia, J. S. Oghalai, and M. J. Justice, “A point mutation in the gene for asparagine-linked glycosylation 10B (Alg10b) causes nonsyndromic hearing impairment in mice (Mus musculus).,” PLoS One, vol. 8, no. 11, p. e80408, 2013.
[203]A. F. Oliveira and R. Yasuda, “An improved Ras sensor for highly sensitive and quantitative FRET-FLIM imaging.,” PLoS One, vol. 8, no. 1, p. e52874, 2013.
[204]B. Nahir and C. E. Jahr, “Activation of Extrasynaptic NMDARs at Individual Parallel Fiber–Molecular Layer Interneuron Synapses in Cerebellum,” The Journal of Neuroscience, vol. 33, no. 41, pp. 16323–16333, 2013.
[205]R. Mostany, J. E. Anstey, K. L. Crump, B. Maco, G. Knott, and C. Portera-Cailliau, “Altered synaptic dynamics during normal brain aging,” The Journal of Neuroscience, vol. 33, no. 9, pp. 4094–4104, 2013.
[206]J. S. Marvin, B. G. Borghuis, L. Tian, J. Cichon, M. T. Harnett, J. Akerboom, A. Gordus, S. L. Renninger, T.-W. Chen, C. I. Bargmann, and others, “An optimized fluorescent probe for visualizing glutamate neurotransmission,” Nature methods, vol. 10, no. 2, pp. 162–170, 2013.
[207]M. S. Maisak, J. Haag, G. Ammer, E. Serbe, M. Meier, A. Leonhardt, T. Schilling, A. Bahl, G. M. Rubin, A. Nern, and others, “A directional tuning map of Drosophila elementary motion detectors,” Nature, vol. 500, no. 7461, pp. 212–216, 2013.
[208]D. Langer, M. van’t Hoff, A. J. Keller, C. Nagaraja, O. A. Pfäffli, M. Göldi, H. Kasper, and F. Helmchen, “HelioScan: A software framework for controlling in vivo microscopy setups with high hardware flexibility, functional diversity and extendibility,” Journal of neuroscience methods, vol. 215, no. 1, pp. 38–52, 2013.
[209]N. G. Horton, K. Wang, D. Kobat, C. G. Clark, F. W. Wise, C. B. Schaffer, and C. Xu, “In vivo three-photon microscopy of subcortical structures within an intact mouse brain,” Nature photonics, vol. 7, no. 3, pp. 205–209, 2013.
[210]C. Hinz, I. Namekawa, J. Behrmann-Godel, C. Oppelt, A. Jaeschke, A. Muller, R. W. Friedrich, and G. Gerlach, “Olfactory imprinting is triggered by MHC peptide ligands.,” Sci Rep, vol. 3, p. 2800, 2013.
[211]T. C. Hill and K. Zito, “LTP-induced long-term stabilization of individual nascent dendritic spines,” The Journal of Neuroscience, vol. 33, no. 2, pp. 678–686, 2013.
[212]M. Hashizume, T. Miyazaki, K. Sakimura, M. Watanabe, K. Kitamura, and M. Kano, “Disruption of cerebellar microzonal organization in GluD2 (GluRdelta2) knockout mouse.,” Front Neural Circuits, vol. 7, p. 130, 2013.
[213]A. E. Granstedt, “In vivo imaging of alphaherpesvirus infection in the mouse peripheral nervous system,” PRINCETON UNIVERSITY, 2013.
[214]O. Fajardo, P. Zhu, and R. W. Friedrich, “Control of a specific motor program by a small brain area in zebrafish.,” Front Neural Circuits, vol. 7, p. 67, 2013.
[215]H. Dehez, M. Piché, and Y. De Koninck, “Resolution and contrast enhancement in laser scanning microscopy using dark beam imaging,” Optics express, vol. 21, no. 13, pp. 15912–15925, 2013.
[216]B. Catalin, D. Alexandru, C. Alub, and M. Iancau, “Microglia branching using a Sholl analysis method,” 2013.
[217]N. Xu, M. T. Harnett, S. R. Williams, D. Huber, D. H. O’Connor, K. Svoboda, and J. C. Magee, “Nonlinear dendritic integration of sensory and motor input during an active sensing task,” Nature, vol. 492, no. 7428, pp. 247–251, Dec. 2012.
[218]M. A. Gelbart, B. He, A. C. Martin, S. Y. Thiberge, E. F. Wieschaus, and M. Kaschube, “Volume conservation principle involved in cell lengthening and nucleus movement during tissue morphogenesis.,” Proc Natl Acad Sci U S A, vol. 109, no. 47, pp. 19298–19303, Nov. 2012.
[219]V. Marra, J. J. Burden, J. R. Thorpe, I. T. Smith, S. L. Smith, M. Hausser, T. Branco, and K. Staras, “A preferentially segregated recycling vesicle pool of limited size supports neurotransmission in native central synapses.,” Neuron, vol. 76, no. 3, pp. 579–589, Nov. 2012.
[220]N. X. Tritsch, J. B. Ding, and B. L. Sabatini, “Dopaminergic neurons inhibit striatal output through non-canonical release of GABA.,” Nature, vol. 490, no. 7419, pp. 262–266, Oct. 2012.
[221]J. Akerboom, T.-W. Chen, T. J. Wardill, L. Tian, J. S. Marvin, S. Mutlu, N. C. Calderon, F. Esposti, B. G. Borghuis, X. R. Sun, A. Gordus, M. B. Orger, R. Portugues, F. Engert, J. J. Macklin, A. Filosa, A. Aggarwal, R. A. Kerr, R. Takagi, S. Kracun, E. Shigetomi, B. S. Khakh, H. Baier, L. Lagnado, S. S.-H. Wang, C. I. Bargmann, B. E. Kimmel, V. Jayaraman, K. Svoboda, D. S. Kim, E. R. Schreiter, and L. L. Looger, “Optimization of a GCaMP calcium indicator for neural activity imaging.,” J Neurosci, vol. 32, no. 40, pp. 13819–13840, Oct. 2012.
[222]J. A. Drocco, E. F. Wieschaus, and D. W. Tank, “The synthesis-diffusion-degradation model explains Bicoid gradient formation in unfertilized eggs.,” Phys Biol, vol. 9, no. 5, p. 055004, Oct. 2012.
[223]L. Petreanu, D. A. Gutnisky, D. Huber, N. Xu, D. H. O’Connor, L. Tian, L. Looger, and K. Svoboda, “Activity in motor-sensory projections reveals distributed coding in somatosensation,” Nature, vol. 489, no. 7415, pp. 299–303, Sep. 2012.
[224]K. A. Buchanan, A. V. Blackman, A. W. Moreau, D. Elgar, R. P. Costa, T. Lalanne, A. A. T. Jones, J. Oyrer, and P. J. Sjöström, “Supplemental Information Target-Specific Expression of Presynaptic NMDA Receptors in Neocortical Microcircuits,” Aug. 2012.
[225]A. Gdalyahu, E. Tring, P.-O. Polack, R. Gruver, P. Golshani, M. S. Fanselow, A. J. Silva, and J. T. Trachtenberg, “Associative fear learning enhances sparse network coding in primary sensory cortex.,” Neuron, vol. 75, no. 1, pp. 121–132, Jul. 2012.
[226]M. Paukert and D. E. Bergles, “Reduction of motion artifacts during in vivo two-photon imaging of brain through heartbeat triggered scanning.,” J Physiol, vol. 590, no. 13, pp. 2955–2963, Jul. 2012.
[227]R. M. Wyatt, E. Tring, and J. T. Trachtenberg, “Pattern and not magnitude of neural activity determines dendritic spine stability in awake mice.,” Nat Neurosci, vol. 15, no. 7, pp. 949–951, Jul. 2012.
[228]K. W. Eliceiri, M. R. Berthold, I. G. Goldberg, L. Ibanez, B. S. Manjunath, M. E. Martone, R. F. Murphy, H. Peng, A. L. Plant, B. Roysam, N. Stuurman, J. R. Swedlow, P. Tomancak, and A. E. Carpenter, “Biological imaging software tools.,” Nat Methods, vol. 9, no. 7, pp. 697–710, Jul. 2012.
[229]Y. Li, H. Lu, P. Cheng, S. Ge, H. Xu, S.-H. Shi, and Y. Dan, “Clonally related visual cortical neurons show similar stimulus feature selectivity.,” Nature, vol. 486, no. 7401, pp. 118–121, Jun. 2012.
[230]A. M. Hamilton, W. C. Oh, H. Vega-Ramirez, I. S. Stein, J. W. Hell, G. N. Patrick, and K. Zito, “Supplemental Information Activity-Dependent Growth of New Dendritic Spines Is Regulated by the Proteasome,” Jun. 2012.
[231]D. Huber, D. A. Gutnisky, S. Peron, D. H. O’Connor, J. S. Wiegert, L. Tian, T. G. Oertner, L. L. Looger, and K. Svoboda, “Multiple dynamic representations in the motor cortex during sensorimotor learning,” Nature, vol. 484, no. 7395, pp. 473–478, Apr. 2012.
[232]C. D. Harvey, P. Coen, and D. W. Tank, “Choice-specific sequences in parietal cortex during a virtual-navigation decision task,” Nature, vol. 484, no. 7392, pp. 62–68, Apr. 2012.
[233]A. H. Morrison, M. Scheeler, J. Dubuis, and T. Gregor, “Quantifying the Bicoid morphogen gradient in living fly embryos.,” Cold Spring Harb Protoc, vol. 2012, no. 4, pp. 398–406, Apr. 2012.
[234]S. Arttamangkul, E. K. Lau, H.-W. Lu, and J. T. Williams, “Desensitization and trafficking of mu-opioid receptors in locus ceruleus neurons: modulation by kinases.,” Mol Pharmacol, vol. 81, no. 3, pp. 348–355, Mar. 2012.
[235]H. A. Zariwala, B. G. Borghuis, T. M. Hoogland, L. Madisen, L. Tian, C. I. De Zeeuw, H. Zeng, L. L. Looger, K. Svoboda, and T.-W. Chen, “A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo.,” J Neurosci, vol. 32, no. 9, pp. 3131–3141, Feb. 2012.
[236]B. Odermatt, A. Nikolaev, and L. Lagnado, “Encoding of luminance and contrast by linear and nonlinear synapses in the retina.,” Neuron, vol. 73, no. 4, pp. 758–773, Feb. 2012.
[237]I. Nauhaus, K. J. Nielsen, and E. M. Callaway, “Nonlinearity of two-photon Ca2+ imaging yields distorted measurements of tuning for V1 neuronal populations.,” J Neurophysiol, vol. 107, no. 3, pp. 923–936, Feb. 2012.
[238]J. B. Ding, W.-J. Oh, B. L. Sabatini, and C. Gu, “Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum.,” Nat Neurosci, vol. 15, no. 2, pp. 215–223, Feb. 2012.
[239]P. Zhu, O. Fajardo, J. Shum, Y.-P. Z. Schärer, and R. W. Friedrich, “High-resolution optical control of spatiotemporal neuronal activity patterns in zebrafish using a digital micromirror device,” Nature protocols, vol. 7, no. 7, pp. 1410–1425, 2012.
[240]M. Wienisch, D. G. Blauvelt, T. F. Sato, and V. N. Murthy, “Two-photon imaging of neural activity in awake, head-restrained mice,” Neuronal Network Analysis: Concepts and Experimental Approaches, pp. 45–60, 2012.
[241]C. Varela, D. A. Llano, and B. B. Theyel, “An Introduction to in vitro slice approaches for the study of neuronal circuitry,” Neuronal Network Analysis: Concepts and Experimental Approaches, pp. 103–125, 2012.
[242]I. Vanzetta, T. Deneux, A. Kaszás, G. Katona, and B. Rozsa, “Functional imaging using two-photon microscopy in living tissue,” Visualization Techniques: From Immunohistochemistry to Magnetic Resonance Imaging, pp. 129–164, 2012.
[243]J. C. Tuthill, Behavioral and electrophysiological investigation of early visual processing in the fly. THE UNIVERSITY OF CHICAGO, 2012.
[244]H. Steffens, F. Nadrigny, and F. Kirchhoff, “In vivo two-photon imaging of neurons and glia in the mouse spinal cord,” Cold Spring Harbor Protocols, vol. 2012, no. 12, p. pdb–prot072264, 2012.
[245]K. M. Seemann, R. Kiefersauer, U. Jacob, and B. Kuhn, “Optical pH detection within a protein crystal,” The Journal of Physical Chemistry B, vol. 116, no. 33, pp. 9873–9881, 2012.
[246]B. B. Scott, T. Gardner, N. Ji, M. S. Fee, and C. Lois, “Wandering neuronal migration in the postnatal vertebrate forebrain,” The Journal of Neuroscience, vol. 32, no. 4, pp. 1436–1446, 2012.
[247]Y.-P. Z. Scharer, J. Shum, A. Moressis, and R. W. Friedrich, “Dopaminergic modulation of synaptic transmission and neuronal activity patterns in the zebrafish homolog of olfactory cortex.,” Front Neural Circuits, vol. 6, p. 76, 2012.
[248]D. R. Rivera, C. M. Brown, D. G. Ouzounov, W. W. Webb, and C. Xu, “Multifocal multiphoton endoscope,” Optics letters, vol. 37, no. 8, pp. 1349–1351, 2012.
[249]S. G. Parra, S. S. Vesuna, T. A. Murray, and M. J. Levene, “YFP ifade Temizlendi Fare Beyin Multiphoton Mikroskopi,” 2012.
[250]S. G. Parra, S. S. Vesuna, T. A. Murray, and M. J. Levene, Multiphoton microscopy of cleared mouse brain expressing YFP. United States, 2012.
[251]I. Ozden, D. A. Dombeck, T. M. Hoogland, D. W. Tank, and S. S.-H. Wang, “Widespread state-dependent shifts in cerebellar activity in locomoting mice.,” PLoS One, vol. 7, no. 8, p. e42650, 2012.
[252]A. I. F. M. de Oliveira, “Regulation of the Ras Pathway by Neurofibromin in Dendritic Spines,” 2012.
[253]C. Nicoletti, N. Offenhauser, D. Jorks, S. Major, and J. P. Dreier, “Assessment of neurovascular coupling,” Animal Models of Acute Neurological Injuries II: Injury and Mechanistic Assessments, Volume 1, pp. 353–372, 2012.
[254]I. Nauhaus, K. J. Nielsen, A. A. Disney, and E. M. Callaway, “Orthogonal micro-organization of orientation and spatial frequency in primate primary visual cortex,” Nature neuroscience, vol. 15, no. 12, pp. 1683–1690, 2012.
[255]B. P. Lehnert, “The Role of TRP Channels in Auditory Transduction and Amplification in Drosophila,” 2012.
[256]B. Kuhn, I. Ozden, Y. Lampi, M. T. Hasan, and S. S.-H. Wang, “An amplified promoter system for targeted expression of calcium indicator proteins in the cerebellar cortex.,” Front Neural Circuits, vol. 6, p. 49, 2012.
[257]N. G. Horton, D. Kobat, K. Wang, and C. Xu, “In Vivo, Deep Tissue Three-Photon Imaging at the 1700-nm Spectral Window,” in Biomedical Optics, 2012, p. BSu2B–2.
[258]A. Holtmaat, V. de Paola, L. Wilbrecht, J. T. Trachtenberg, K. Svoboda, and C. Portera-Cailliau, “Imaging neocortical neurons through a chronic cranial window,” Cold Spring Harbor Protocols, vol. 2012, no. 6, p. pdb–prot069617, 2012.
[259]J. A. Drocco, E. F. Wieschaus, and D. W. Tank, “The synthesis–diffusion–degradation model explains Bicoid gradient formation in unfertilized eggs,” Physical biology, vol. 9, no. 5, p. 055004, 2012.
[260]A. Cruz-Martin, M. Crespo, and C. Portera-Cailliau, “Glutamate induces the elongation of early dendritic protrusions via mGluRs in wild type mice, but not in fragile X mice.,” PLoS One, vol. 7, no. 2, p. e32446, 2012.
[261]S. T. Bundschuh, P. Zhu, Y.-P. Z. Schärer, and R. W. Friedrich, “Dopaminergic modulation of mitral cells and odor responses in the zebrafish olfactory bulb,” The Journal of Neuroscience, vol. 32, no. 20, pp. 6830–6840, 2012.
[262]J. M. Bélisle, “Développement et caractérisation d’une méthode photonique pour créer des distributions spatiales de protéines,” 2012.
[263]S. Arttamangkul, E. K. Lau, H.-W. Lu, and J. T. Williams, “Desensitization and trafficking of μ-opioid receptors in locus ceruleus neurons: modulation by kinases,” Molecular pharmacology, vol. 81, no. 3, pp. 348–355, 2012.
[264]M. L. Andermann, A. M. Kerlin, D. K. Roumis, L. L. Glickfeld, and R. C. Reid, “Functional specialization of mouse higher visual cortical areas.,” Neuron, vol. 72, no. 6, pp. 1025–1039, Dec. 2011.
[265]T. Baden, F. Esposti, A. Nikolaev, and L. Lagnado, “Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision.,” Curr Biol, vol. 21, no. 22, pp. 1859–1869, Nov. 2011.
[266]J. R. Pugh and C. E. Jahr, “NMDA receptor agonists fail to alter release from cerebellar basket cells.,” J Neurosci, vol. 31, no. 46, pp. 16550–16555, Nov. 2011.
[267]C. C. Liu, S. S. Gao, T. Yuan, C. Steele, S. Puria, and J. S. Oghalai, “Biophysical mechanisms underlying outer hair cell loss associated with a shortened tectorial membrane.,” J Assoc Res Otolaryngol, vol. 12, no. 5, pp. 577–594, Oct. 2011.
[268]G. F. Woods, W. C. Oh, L. C. Boudewyn, S. K. Mikula, and K. Zito, “Loss of PSD-95 enrichment is not a prerequisite for spine retraction.,” J Neurosci, vol. 31, no. 34, pp. 12129–12138, Aug. 2011.
[269]K. Kitamura and M. Hausser, “Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo.,” J Neurosci, vol. 31, no. 30, pp. 10847–10858, Jul. 2011.
[270]A. J. Ramsey, M. Milenkovic, A. F. Oliveira, Y. Escobedo-Lozoya, S. Seshadri, A. Salahpour, A. Sawa, R. Yasuda, and M. G. Caron, “Impaired NMDA receptor transmission alters striatal synapses and DISC1 protein in an age-dependent manner.,” Proc Natl Acad Sci U S A, vol. 108, no. 14, pp. 5795–5800, Apr. 2011.
[271]B. G. Borghuis, L. Tian, Y. Xu, S. S. Nikonov, N. Vardi, B. V. Zemelman, and L. L. Looger, “Imaging light responses of targeted neuron populations in the rodent retina.,” J Neurosci, vol. 31, no. 8, pp. 2855–2867, Feb. 2011.
[272]R. Mostany and C. Portera-Cailliau, “Absence of large-scale dendritic plasticity of layer 5 pyramidal neurons in peri-infarct cortex,” J. Neurosci., vol. 31, no. 5, pp. 1734–1738, Feb. 2011.
[273]J. R. Pugh and C. E. Jahr, “Axonal GABAA receptors increase cerebellar granule cell excitability and synaptic activity.,” J Neurosci, vol. 31, no. 2, pp. 565–574, Jan. 2011.
[274]R. A. Weber, C. Rodriguez, D. N. Nguyen, L. A. Emmert, W. Rudolph, D. Patel, and C. S. Menoni, “Third harmonic microscopy for optical material characterization,” in XLIII Annual Symposium on Optical Materials for High Power Lasers, 2011, p. 81900V–81900V.
[275]S. Vesuna, R. Torres, and M. J. Levene, “Multiphoton fluorescence, second harmonic generation, and fluorescence lifetime imaging of whole cleared mouse organs,” Journal of biomedical optics, vol. 16, no. 10, pp. 106009–106009, 2011.
[276]T. V. Truong, W. Supatto, D. S. Koos, J. M. Choi, and S. E. Fraser, “Deep and fast live imaging with two-photon scanned light-sheet microscopy,” Nature Methods, vol. 8, no. 9, pp. 757–760, 2011.
[277]P. Schönenberger Lawrence, “Optogenetic approaches to the study of hippocampal long-term plasticity,” University_of_Basel, 2011.
[278]H. Murakoshi, H. Wang, and R. Yasuda, “Local, persistent activation of Rho GTPases during plasticity of single dendritic spines,” Nature, vol. 472, no. 7341, pp. 100–104, 2011.
[279]L. B. Mostaço-Guidolin, A. C. Ko, D. P. Popescu, M. S. Smith, E. K. Kohlenberg, M. Shiomi, A. Major, and M. G. Sowa, “Evaluation of texture parameters for the quantitative description of multimodal nonlinear optical images from atherosclerotic rabbit arteries,” Physics in medicine and biology, vol. 56, no. 16, p. 5319, 2011.
[280]S.-J. Lee, “Spatiotemporal Dynamics of,” Duke University, 2011.
[281]S. J. Kuhlman, E. Tring, and J. T. Trachtenberg, “Fast-spiking interneurons have an initial orientation bias that is lost with vision,” Nature neuroscience, vol. 14, no. 9, pp. 1121–1123, 2011.
[282]E. Herzog, F. Nadrigny, K. Silm, C. Biesemann, I. Helling, T. Bersot, H. Steffens, R. Schwartzmann, U. V. Nägerl, S. El Mestikawy, and others, “In vivo imaging of intersynaptic vesicle exchange using VGLUT1Venus knock-in mice,” The Journal of Neuroscience, vol. 31, no. 43, pp. 15544–15559, 2011.
[283]M. A. Herman, B. Nahir, and C. E. Jahr, “Distribution of extracellular glutamate in the neuropil of hippocampus.,” PLoS One, vol. 6, no. 11, p. e26501, 2011.
[284]M. I. Hamad, Z.-L. Ma-Högemeier, C. Riedel, C. Conrads, T. Veitinger, T. Habijan, J.-N. Schulz, M. Krause, M. J. Wirth, M. Hollmann, and others, “Cell class-specific regulation of neocortical dendrite and spine growth by AMPA receptor splice and editing variants,” Development, vol. 138, no. 19, pp. 4301–4313, 2011.
[285]E. F. Civillico, J. P. Rickgauer, and S. S.-H. Wang, “Targeting and excitation of photoactivatable molecules: design considerations for neurophysiology experiments,” Photosensitive Molecules for Controlling Biological Function, pp. 7–37, 2011.
[286]F. Blumhagen, P. Zhu, J. Shum, Y.-P. Z. Schärer, E. Yaksi, K. Deisseroth, and R. W. Friedrich, “Neuronal filtering of multiplexed odour representations,” Nature, vol. 479, no. 7374, pp. 493–498, 2011.
[287]S. Begin, B. Burgoyne, V. Mercier, A. Villeneuve, R. Vallee, and D. Cote, “Coherent anti-Stokes Raman scattering hyperspectral tissue imaging with a wavelength-swept system.,” Biomed Opt Express, vol. 2, no. 5, pp. 1296–1306, 2011.
[288]S. M. Martin, G. S. O’Brien, C. Portera-Cailliau, and A. Sagasti, “Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning.,” Development, vol. 137, no. 23, pp. 3985–3994, Dec. 2010.
[289]D. A. Dombeck, C. D. Harvey, L. Tian, L. L. Looger, and D. W. Tank, “Functional imaging of hippocampal place cells at cellular resolution during virtual navigation,” Nat. Neurosci., vol. 13, no. 11, pp. 1433–1440, Nov. 2010.
[290]A. M. Kerlin, M. L. Andermann, V. K. Berezovskii, and R. C. Reid, “Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex.,” Neuron, vol. 67, no. 5, pp. 858–871, Sep. 2010.
[291]M. A. Patterson, E. M. Szatmari, and R. Yasuda, “AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation.,” Proc Natl Acad Sci U S A, vol. 107, no. 36, pp. 15951–15956, Sep. 2010.
[292]M. J. Higley and B. L. Sabatini, “Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors.,” Nat Neurosci, vol. 13, no. 8, pp. 958–966, Aug. 2010.
[293]J. D. Seelig, M. E. Chiappe, G. K. Lott, A. Dutta, J. E. Osborne, M. B. Reiser, and V. Jayaraman, “Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior,” Nat. Methods, vol. 7, no. 7, pp. 535–540, Jul. 2010.
[294]A. Cruz-Martin, M. Crespo, and C. Portera-Cailliau, “Delayed stabilization of dendritic spines in fragile X mice.,” J Neurosci, vol. 30, no. 23, pp. 7793–7803, Jun. 2010.
[295]M. M. Dorostkar, E. Dreosti, B. Odermatt, and L. Lagnado, “Computational processing of optical measurements of neuronal and synaptic activity in networks.,” J Neurosci Methods, vol. 188, no. 1, pp. 141–150, Apr. 2010.
[296]L. Wilbrecht, A. Holtmaat, N. Wright, K. Fox, and K. Svoboda, “Structural plasticity underlies experience-dependent functional plasticity of cortical circuits.,” J Neurosci, vol. 30, no. 14, pp. 4927–4932, Apr. 2010.
[297]A. Xia, S. S. Gao, T. Yuan, A. Osborn, A. Bress, M. Pfister, S. M. Maricich, F. A. Pereira, and J. S. Oghalai, “Deficient forward transduction and enhanced reverse transduction in the alpha tectorin C1509G human hearing loss mutation.,” Dis Model Mech, vol. 3, no. 3–4, pp. 209–223, Apr. 2010.
[298]M. T. Heneka, F. Nadrigny, T. Regen, A. Martinez-Hernandez, L. Dumitrescu-Ozimek, D. Terwel, D. Jardanhazi-Kurutz, J. Walter, F. Kirchhoff, U.-K. Hanisch, and M. P. Kummer, “Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine,” Proc. Natl. Acad. Sci. U.S.A., vol. 107, no. 13, pp. 6058–6063, Mar. 2010.
[299]T. Yuan, S. S. Gao, P. Saggau, and J. S. Oghalai, “Calcium imaging of inner ear hair cells within the cochlear epithelium of mice using two-photon microscopy.,” J Biomed Opt, vol. 15, no. 1, p. 016002, Feb. 2010.
[300]B. A. Suter, T. O’Connor, V. Iyer, L. T. Petreanu, B. M. Hooks, T. Kiritani, K. Svoboda, and G. M. G. Shepherd, “Ephus: multipurpose data acquisition software for neuroscience experiments.,” Front Neural Circuits, vol. 4, p. 100, 2010.
[301]T. R. Sato and K. Svoboda, “The functional properties of barrel cortex neurons projecting to the primary motor cortex,” The Journal of Neuroscience, vol. 30, no. 12, pp. 4256–4260, 2010.
[302]M. A. Patterson, E. M. Szatmari, and R. Yasuda, “AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK–dependent manner during long-term potentiation,” Proceedings of the National Academy of Sciences, vol. 107, no. 36, pp. 15951–15956, 2010.
[303]S. G. Parra, T. H. Chia, J. P. Zinter, and M. J. Levene, “Multiphoton microscopy of cleared mouse organs,” Journal of biomedical optics, vol. 15, no. 3, pp. 036017–036017, 2010.
[304]D. H. O’Connor, S. P. Peron, D. Huber, and K. Svoboda, “Neural activity in barrel cortex underlying vibrissa-based object localization in mice,” Neuron, vol. 67, no. 6, pp. 1048–1061, 2010.
[305]J. Niessing and R. W. Friedrich, “Olfactory pattern classification by discrete neuronal network states,” Nature, vol. 465, no. 7294, pp. 47–52, 2010.
[306]L. B. Mostaco-Guidolin, “Multimodal characterization of atherosclerotic cardiovascular disease with label-free non-linear optical imaging techniques,” 2010.
[307]L. B. Mostaco-Guidolin, M. G. Sowa, A. Ridsdale, A. F. Pegoraro, M. S. D. Smith, M. D. Hewko, E. K. Kohlenberg, B. Schattka, M. Shiomi, A. Stolow, and A. C.-T. Ko, “Differentiating atherosclerotic plaque burden in arterial tissues using femtosecond CARS-based multimodal nonlinear optical imaging.,” Biomed Opt Express, vol. 1, no. 1, pp. 59–73, 2010.
[308]J. H. Marshel, T. Mori, K. J. Nielsen, and E. M. Callaway, “Targeting single neuronal networks for gene expression and cell labeling in vivo,” Neuron, vol. 67, no. 4, pp. 562–574, 2010.
[309]A. C. Ko, A. Ridsdale, M. S. Smith, L. B. Mostaço-Guidolin, M. D. Hewko, A. F. Pegoraro, E. K. Kohlenberg, B. Schattka, M. Shiomi, A. Stolow, and others, “Multimodal nonlinear optical imaging of atherosclerotic plaque development in myocardial infarction-prone rabbits,” Journal of biomedical optics, vol. 15, no. 2, pp. 020501–020501, 2010.
[310]N. Holbro, “Structure-function analysis on the level of individual synapses,” University_of_Basel, 2010.
[311]B. Heider, J. L. Nathanson, E. Y. Isacoff, E. M. Callaway, and R. M. Siegel, “Two-photon imaging of calcium in virally transfected striate cortical neurons of behaving monkey.,” PLoS One, vol. 5, no. 11, p. e13829, 2010.
[312]J. Dubuis, A. H. Morrison, M. Scheeler, and T. Gregor, “Quantifying the Bicoid morphogen gradient in living fly embryos,” arXiv preprint arXiv:1003.5572, 2010.
[313]T. G. Chowdhury, J. C. Jimenez, J. M. Bomar, A. Cruz-Martin, J. P. Cantle, and C. Portera-Cailliau, “Fate of cajal-retzius neurons in the postnatal mouse neocortex.,” Front Neuroanat, vol. 4, p. 10, 2010.
[314]S. Chen, X. Feng, Y. Li, C. Zhou, P. Xi, and Q. Ren, “Software controlling algorithms for the system performance optimization of confocal laser scanning microscope,” Biomedical Signal Processing and Control, vol. 5, no. 3, pp. 223–228, 2010.
[315]B. A. Ashcroft and T. Oosterkamp, “AutoMicromanager: A microscopy scripting toolkit for LABVIEW and other programming environments,” Review of Scientific Instruments, vol. 81, no. 11, p. 113708, 2010.
[316]M. L. Andermann, A. M. Kerlin, and R. C. Reid, “Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing,” Front Cell Neurosci, vol. 4, p. 3, 2010.
[317]G. S. O’Brien, S. M. Martin, C. Söllner, G. J. Wright, C. G. Becker, C. Portera-Cailliau, and A. Sagasti, “Supplemental Data Developmentally Regulated Impediments to Skin Reinnervation by Injured Peripheral Sensory Axon Terminals,” Dec. 2009.
[318]C. Matter, M. Pribadi, X. Liu, and J. T. Trachtenberg, “Delta-catenin is required for the maintenance of neural structure and function in mature cortex in vivo,” Neuron, vol. 64, no. 3, pp. 320–327, Nov. 2009.
[319]J. M. Christie and C. E. Jahr, “Selective expression of ligand-gated ion channels in L5 pyramidal cell axons.,” J Neurosci, vol. 29, no. 37, pp. 11441–11450, Sep. 2009.
[320]P. Golshani, J. T. Goncalves, S. Khoshkhoo, R. Mostany, S. Smirnakis, and C. Portera-Cailliau, “Internally mediated developmental desynchronization of neocortical network activity.,” J Neurosci, vol. 29, no. 35, pp. 10890–10899, Sep. 2009.
[321]N. Holbro, A. Grunditz, and T. G. Oertner, “Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses.,” Proc Natl Acad Sci U S A, vol. 106, no. 35, pp. 15055–15060, Sep. 2009.
[322]M. J. Higley, G. J. Soler-Llavina, and B. L. Sabatini, “Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration.,” Nat Neurosci, vol. 12, no. 9, pp. 1121–1128, Sep. 2009.
[323]S. B. Simons, Y. Escobedo, R. Yasuda, and S. M. Dudek, “Regional differences in hippocampal calcium handling provide a cellular mechanism for limiting plasticity.,” Proc Natl Acad Sci U S A, vol. 106, no. 33, pp. 14080–14084, Aug. 2009.
[324]M. S. Virk, S. Arttamangkul, W. T. Birdsong, and J. T. Williams, “Buprenorphine is a weak partial agonist that inhibits opioid receptor desensitization.,” J Neurosci, vol. 29, no. 22, pp. 7341–7348, Jun. 2009.
[325]H. Zhong, G.-M. Sia, T. R. Sato, N. W. Gray, T. Mao, Z. Khuchua, R. L. Huganir, and K. Svoboda, “Subcellular dynamics of type II PKA in neurons.,” Neuron, vol. 62, no. 3, pp. 363–374, May 2009.
[326]T. M. Hoogland, B. Kuhn, W. Gobel, W. Huang, J. Nakai, F. Helmchen, J. Flint, and S. S.-H. Wang, “Radially expanding transglial calcium waves in the intact cerebellum.,” Proc Natl Acad Sci U S A, vol. 106, no. 9, pp. 3496–3501, Mar. 2009.
[327]P. S. Tsai and D. Kleinfeld, “3 In Vivo Two-Photon Laser Scanning Microscopy with Concurrent Plasma-Mediated Ablation,” Methods for In Vivo Optical Imaging, vol. 3, pp. 59–115, Mar. 2009.
[328]T.-P. L. Scanning and P.-M. Ablation, “4 MPScope 2.0,” Mar. 2009.
[329]K. Zito, V. Scheuss, G. Knott, T. Hill, and K. Svoboda, “Rapid functional maturation of nascent dendritic spines.,” Neuron, vol. 61, no. 2, pp. 247–258, Jan. 2009.
[330]T. Yuan, S. S. Gao, P. Saggau, and J. S. Oghalai, “Imaging living hair cells within the cochlear epithelium of mice using two-photon microscopy,” in SPIE BiOS: Biomedical Optics, 2009, pp. 718209–718209.
[331]Y. J. Yu, S. Arttamangkul, C. J. Evans, J. T. Williams, and M. von Zastrow, “Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of μ-opioid receptors in CNS neurons,” The Journal of Neuroscience, vol. 29, no. 1, pp. 222–233, 2009.
[332]M. S. Smith, A. C. Ko, A. Ridsdale, B. Schattka, A. Pegoraro, M. D. Hewko, M. Shiomi, A. Stolow, and M. G. Sowa, “A single-photon fluorescence and multi-photon spectroscopic study of atherosclerotic lesions,” in Photonics North 2009, 2009, p. 73860I–73860I.
[333]S. R. Schultz, K. Kitamura, A. Post-Uiterweer, J. Krupic, and M. Häusser, “Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells,” The Journal of Neuroscience, vol. 29, no. 25, pp. 8005–8015, 2009.
[334]P. Schoenenberger, D. Gerosa, and T. G. Oertner, “Temporal control of immediate early gene induction by light.,” PLoS One, vol. 4, no. 12, p. e8185, 2009.
[335]G. S. O’Brien, S. Rieger, S. M. Martin, A. M. Cavanaugh, C. Portera-Cailliau, and A. Sagasti, “Two-photon axotomy and time-lapse confocal imaging in live zebrafish embryos,” J Vis Exp, vol. 24, no. 1129.001, pp. 10–3791, 2009.
[336]C. Matter, M. Pribadi, X. Liu, and J. T. Trachtenberg, “δ-catenin is required for the maintenance of neural structure and function in mature cortex in vivo,” Neuron, vol. 64, no. 3, pp. 320–327, 2009.
[337]B. Judkewitz, M. Rizzi, K. Kitamura, and M. Häusser, “Targeted single-cell electroporation of mammalian neurons in vivo,” Nature protocols, vol. 4, no. 6, pp. 862–869, 2009.
[338]A. Holtmaat, T. Bonhoeffer, D. K. Chow, J. Chuckowree, V. De Paola, S. B. Hofer, M. Hubener, T. Keck, G. Knott, W.-C. A. Lee, R. Mostany, T. D. Mrsic-Flogel, E. Nedivi, C. Portera-Cailliau, K. Svoboda, J. T. Trachtenberg, and L. Wilbrecht, “Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window.,” Nat Protoc, vol. 4, no. 8, pp. 1128–1144, 2009.
[339]M. A. Herman, “Control of extracellular glutamate by transporters in the CNS,” 2009.
[340]R. D. Frostig, P. S. Tsai, and D. Kleinfeld, “In vivo two-photon laser scanning microscopy with concurrent plasma-mediated ablation principles and hardware realization,” 2009.
[341]R. D. Frostig, Q.-T. Nguyen, J. Driscoll, E. M. Dolnick, and D. Kleinfeld, “MPScope 2.0: A computer system for two-photon laser scanning microscopy with concurrent plasma-mediated ablation and electrophysiology,” 2009.
[342]J. Flinta and S. S.-H. Wanga, “Radially expanding transglial calcium waves in the intact cerebellum,” PNAS, vol. 106, no. 9, p. 3497, 2009.
[343]J. B. Ding, K. T. Takasaki, and B. L. Sabatini, “Supraresolution imaging in brain slices using stimulated-emission depletion two-photon laser scanning microscopy,” Neuron, vol. 63, no. 4, pp. 429–437, 2009.
[344]T. H. Chia and M. J. Levene, “Microprisms for in vivo multilayer cortical imaging,” Journal of neurophysiology, vol. 102, no. 2, pp. 1310–1314, 2009.
[345]E. Casanova, N. Guetg, R. Vigot, R. Seddik, M. Julio-Pieper, N. P. Hyland, J. F. Cryan, M. Gassmann, and B. Bettler, “A mouse model for visualization of GABAB receptors,” genesis, vol. 47, no. 9, pp. 595–602, 2009.
[346]A. Bullen, R. S. Friedman, and M. F. Krummel, “Two-photon imaging of the immune system: a custom technology platform for high-speed, multicolor tissue imaging of immune responses,” in Visualizing Immunity, Springer, 2009, pp. 1–29.
[347]C. D. Harvey, A. G. Ehrhardt, C. Cellurale, H. Zhong, R. Yasuda, R. J. Davis, and K. Svoboda, “A genetically encoded fluorescent sensor of ERK activity.,” Proc Natl Acad Sci U S A, vol. 105, no. 49, pp. 19264–19269, Dec. 2008.
[348]A. W. Bigelow, C. R. Geard, G. Randers-Pehrson, and D. J. Brenner, “Microbeam-integrated multiphoton imaging system.,” Rev Sci Instrum, vol. 79, no. 12, p. 123707, Dec. 2008.
[349]S. P. Gandhi, Y. Yanagawa, and M. P. Stryker, “Delayed plasticity of inhibitory neurons in developing visual cortex.,” Proc Natl Acad Sci U S A, vol. 105, no. 43, pp. 16797–16802, Oct. 2008.
[350]J. M. Christie and C. E. Jahr, “Dendritic NMDA receptors activate axonal calcium channels.,” Neuron, vol. 60, no. 2, pp. 298–307, Oct. 2008.
[351]S. Arttamangkul, N. Quillinan, M. J. Low, M. von Zastrow, J. Pintar, and J. T. Williams, “Differential activation and trafficking of micro-opioid receptors in brain slices.,” Mol Pharmacol, vol. 74, no. 4, pp. 972–979, Oct. 2008.
[352]A. C. Riegel and J. T. Williams, “CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons.,” Neuron, vol. 57, no. 4, pp. 559–570, Feb. 2008.
[353]D. Huber, L. Petreanu, N. Ghitani, S. Ranade, T. Hromadka, Z. Mainen, and K. Svoboda, “Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice.,” Nature, vol. 451, no. 7174, pp. 61–64, Jan. 2008.
[354]AAsa Müller-Grunditz, “Dendritic spines as chemical and electrical compartments: a two-photon imaging study in the hippocampus of the rat,” University_of_Basel, 2008.
[355]R. Mostany and C. Portera-Cailliau, “A method for 2-photon imaging of blood flow in the neocortex through a cranial window,” J Vis Exp, vol. 12, 2008.
[356]T. Mao, D. H. O’Connor, V. Scheuss, J. Nakai, and K. Svoboda, “Characterization and subcellular targeting of GCaMP-type genetically-encoded calcium indicators.,” PLoS One, vol. 3, no. 3, p. e1796, 2008.
[357]G. Major, A. Polsky, W. Denk, J. Schiller, and D. W. Tank, “Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons,” Journal of neurophysiology, vol. 99, no. 5, pp. 2584–2601, 2008.
[358]S. J. Kuhlman and Z. J. Huang, “High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression.,” PLoS One, vol. 3, no. 4, p. e2005, 2008.
[359]M. Krumin and S. Shoham, “Characterization of input-output relations in single neurons using spatiotemporal photo-stimulation,” in 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, 2008, pp. 378–379.
[360]AAsa Grunditz, N. Holbro, L. Tian, Y. Zuo, and T. G. Oertner, “Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization,” The Journal of neuroscience, vol. 28, no. 50, pp. 13457–13466, 2008.
[361]P. Golshani and C. Portera-Cailliau, “In vivo 2-photon calcium imaging in layer 2/3 of mice,” Journal of visualized experiments: JoVE, no. 13, 2008.
[362]S. Arttamangkul, N. Quillinan, M. J. Low, M. Von Zastrow, J. Pintar, and J. T. Williams, “Differential activation and trafficking of μ-opioid receptors in brain slices,” Molecular pharmacology, vol. 74, no. 4, pp. 972–979, 2008.
[363]C. D. Harvey and K. Svoboda, “Locally dynamic synaptic learning rules in pyramidal neuron dendrites.,” Nature, vol. 450, no. 7173, pp. 1195–1200, Dec. 2007.
[364]D. A. Dombeck, A. N. Khabbaz, F. Collman, T. L. Adelman, and D. W. Tank, “Imaging large-scale neural activity with cellular resolution in awake, mobile mice,” Neuron, vol. 56, no. 1, pp. 43–57, Oct. 2007.
[365]E. M. C. Hillman, “Optical brain imaging in vivo: techniques and applications from animal to man.,” J Biomed Opt, vol. 12, no. 5, p. 051402, Oct. 2007.
[366]T. Gregor, D. W. Tank, E. F. Wieschaus, and W. Bialek, “Probing the limits to positional information.,” Cell, vol. 130, no. 1, pp. 153–164, Jul. 2007.
[367]A. Sobczyk and K. Svoboda, “Activity-dependent plasticity of the NMDA-receptor fractional Ca 2+ current,” Neuron, vol. 53, no. 1, pp. 17–24, 2007.
[368]T. R. Sato, N. W. Gray, Z. F. Mainen, and K. Svoboda, “The functional microarchitecture of the mouse barrel cortex,” PLoS Biol, vol. 5, no. 7, p. e189, 2007.
[369]M. Z. Nazir, A High Speed Multi-channel Time-correlated System for Fluorescence Lifetime Imaging. ProQuest, 2007.
[370]T. H. Chia, A. Williamson, D. D. Spencer, and M. J. Levene, “Multiphoton Fluorescence Imaging of NADH to Investigate Metabolic Changes in Human Epileptic Tissue; in vitro,” in Conference on Lasers and Electro-Optics, 2007, p. CTuP1.
[371]A. G. Carter, G. J. Soler-Llavina, and B. L. Sabatini, “Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons,” The Journal of Neuroscience, vol. 27, no. 33, pp. 8967–8977, 2007.
[372]T. Ye, G. Yurtsever, M. Fischer, J. D. Simon, and W. S. Warren, “Imaging melanin by two-photon absorption microscopy,” in Biomedical Optics 2006, 2006, p. 60891X–60891X.
[373]R. Yasuda, C. D. Harvey, H. Zhong, A. Sobczyk, L. Van Aelst, and K. Svoboda, “Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging,” Nature neuroscience, vol. 9, no. 2, pp. 283–291, 2006.
[374]R. Vigot, S. Barbieri, H. Bräuner-Osborne, R. Turecek, R. Shigemoto, Y.-P. Zhang, R. Luján, L. H. Jacobson, B. Biermann, J.-M. Fritschy, and others, “Differential compartmentalization and distinct functions of GABA B receptor variants,” Neuron, vol. 50, no. 4, pp. 589–601, 2006.
[375]K. Svoboda and R. Yasuda, “Principles of two-photon excitation microscopy and its applications to neuroscience,” Neuron, vol. 50, no. 6, pp. 823–839, 2006.
[376]G. J. Soler-Llavina and B. L. Sabatini, “Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells,” Nature neuroscience, vol. 9, no. 6, pp. 798–806, 2006.
[377]P. J. Sjöström and M. Häusser, “A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons,” Neuron, vol. 51, no. 2, pp. 227–238, 2006.
[378]V. Scheuss, R. Yasuda, A. Sobczyk, and K. Svoboda, “Nonlinear [Ca2+] signaling in dendrites and spines caused by activity-dependent depression of Ca2+ extrusion,” The Journal of neuroscience, vol. 26, no. 31, pp. 8183–8194, 2006.
[379]D. V. Sarkisov, “Multiphoton Approaches to Learning Rules in the Cerebellum: Using Light to Manipulate Biochemistry,” Princeton University, 2006.
[380]P. G. Papageorgas, D. Maroulis, G. Anagnostopoulos, H. Albrecht, B. Wagner, D. K. Iakovidis, and N. G. Theofanous, “A high-performance imaging and control system for a micromirror-based laser-scanning endoscope device,” Instrumentation and Measurement, IEEE Transactions on, vol. 55, no. 5, pp. 1725–1733, 2006.
[381]M. Oheim, D. J. Michael, M. Geisbauer, D. Madsen, and R. H. Chow, “Principles of two-photon excitation fluorescence microscopy and other nonlinear imaging approaches,” Advanced drug delivery reviews, vol. 58, no. 7, pp. 788–808, 2006.
[382]Q.-T. Nguyen, P. S. Tsai, and D. Kleinfeld, “MPScope: a versatile software suite for multiphoton microscopy,” Journal of neuroscience methods, vol. 156, no. 1, pp. 351–359, 2006.
[383]A. Holtmaat, L. Wilbrecht, G. W. Knott, E. Welker, and K. Svoboda, “Experience-dependent and cell-type-specific spine growth in the neocortex,” Nature, vol. 441, no. 7096, pp. 979–983, 2006.
[384]N. W. Gray, R. M. Weimer, I. Bureau, and K. Svoboda, “Rapid redistribution of synaptic PSD-95 in the neocortex in vivo,” PLoS Biol, vol. 4, no. 11, p. e370, 2006.
[385]V. De Paola, A. Holtmaat, G. Knott, S. Song, L. Wilbrecht, P. Caroni, and K. Svoboda, “Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex,” Neuron, vol. 49, no. 6, pp. 861–875, 2006.
[386]A. Birbach, J. M. Verkuyl, and A. Matus, “Reversible, activity-dependent targeting of profilin to neuronal nuclei,” Experimental cell research, vol. 312, no. 12, pp. 2279–2287, 2006.
[387]S. Arttamangkul, M. Torrecilla, K. Kobayashi, H. Okano, and J. T. Williams, “Separation of μ-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures,” The Journal of neuroscience, vol. 26, no. 15, pp. 4118–4125, 2006.
[388]F. Helmchen and W. Denk, “Deep tissue two-photon microscopy,” Nat. Methods, vol. 2, no. 12, pp. 932–940, Dec. 2005.
[389]A. Sobczyk, V. Scheuss, and K. Svoboda, “NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines,” The Journal of neuroscience, vol. 25, no. 26, pp. 6037–6046, 2005.
[390]B. L. Sabatini, “The Role of TSC1 in the Formation and Maintenance of Excitatory Synapses,” DTIC Document, 2005.
[391]C. Portera-Cailliau, R. M. Weimer, V. De Paola, P. Caroni, and K. Svoboda, “Diverse modes of axon elaboration in the developing neocortex,” PLoS Biol, vol. 3, no. 8, p. e272, 2005.
[392]K. Ohki, S. Chung, Y. H. Ch’ng, P. Kara, and R. C. Reid, “Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex,” Nature, vol. 433, no. 7026, pp. 597–603, 2005.
[393]T. J. Ngo-Anh, B. L. Bloodgood, M. Lin, B. L. Sabatini, J. Maylie, and J. P. Adelman, “SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines,” Nature neuroscience, vol. 8, no. 5, pp. 642–649, 2005.
[394]A. J. Holtmaat, J. T. Trachtenberg, L. Wilbrecht, G. M. Shepherd, X. Zhang, G. W. Knott, and K. Svoboda, “Transient and persistent dendritic spines in the neocortex in vivo,” Neuron, vol. 45, no. 2, pp. 279–291, 2005.
[395]V. Egger, K. Svoboda, and Z. F. Mainen, “Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike,” The journal of neuroscience, vol. 25, no. 14, pp. 3521–3530, 2005.
[396]K. Zito, G. Knott, G. M. Shepherd, S. Shenolikar, and K. Svoboda, “Induction of spine growth and synapse formation by regulation of the spine actin cytoskeleton,” Neuron, vol. 44, no. 2, pp. 321–334, 2004.
[397]R. Yasuda, E. A. Nimchinsky, V. Scheuss, T. A. Pologruto, T. G. Oertner, B. L. Sabatini, and K. Svoboda, “Imaging calcium concentration dynamics in small neuronal compartments,” Sci STKE, vol. 2004, no. 219, p. pl5, 2004.
[398]Y. Wang, H.-F. Guo, T. A. Pologruto, F. Hannan, I. Hakker, K. Svoboda, and Y. Zhong, “Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging,” The Journal of neuroscience, vol. 24, no. 29, pp. 6507–6514, 2004.
[399]T. A. Pologruto, R. Yasuda, and K. Svoboda, “Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators,” The Journal of neuroscience, vol. 24, no. 43, pp. 9572–9579, 2004.
[400]E. A. Nimchinsky, R. Yasuda, T. G. Oertner, and K. Svoboda, “The number of glutamate receptors opened by synaptic stimulation in single hippocampal spines,” The Journal of neuroscience, vol. 24, no. 8, pp. 2054–2064, 2004.
[401]A. G. Carter and B. L. Sabatini, “State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons,” Neuron, vol. 44, no. 3, pp. 483–493, 2004.
[402]I. Brünig, S. Kaech, H. Brinkhaus, T. G. Oertner, and A. Matus, “Influx of extracellular calcium regulates actin-dependent morphological plasticity in dendritic spines,” Neuropharmacology, vol. 47, no. 5, pp. 669–676, 2004.
[403]R. Yasuda, B. L. Sabatini, and K. Svoboda, “Plasticity of calcium channels in dendritic spines,” Nature neuroscience, vol. 6, no. 9, pp. 948–955, 2003.
[404]V. Egger, K. Svoboda, and Z. F. Mainen, “Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells,” The Journal of neuroscience, vol. 23, no. 20, pp. 7551–7558, 2003.